The aim of this international comparison was to compare treatment strategies and relative survival between countries for older patients with colon cancer, using data from population‐based cancer registries across Europe.
Keywords: Aged 80 and over, Colonic neoplasms, Europe, Surgery, Adjuvant chemotherapy, Survival
Abstract
Background.
Colon cancer in older patients represents a major public health issue. As older patients are hardly included in clinical trials, the optimal treatment of these patients remains unclear. The present international EURECCA comparison explores possible associations between treatment and survival outcomes in elderly colon cancer patients.
Subjects, Materials, and Methods.
National data from Belgium, Denmark, The Netherlands, Norway, and Sweden were obtained, as well as a multicenter surgery cohort from Germany. Patients aged 80 years and older, diagnosed with colon cancer between 2001 and 2010, were included. The study interval was divided into two periods: 2001–2006 and 2007–2010. The proportion of surgical treatment and chemotherapy within a country and its relation to relative survival were calculated for each time frame.
Results.
Overall, 50,761 patients were included. At least 94% of patients with stage II and III colon cancer underwent surgical removal of the tumor. For stage II–IV, the proportion of chemotherapy after surgery was highest in Belgium and lowest in The Netherlands and Norway. For stage III, it varied from 24.8% in Belgium and 3.9% in Norway. For stage III, a better adjusted relative survival between 2007 and 2010 was observed in Sweden (adjusted relative excess risk [RER] 0.64, 95% confidence interval [CI]: 0.54–0.76) and Norway (adjusted RER 0.81, 95% CI: 0.69–0.96) compared with Belgium.
Conclusion.
There is substantial variation in the rate of treatment and survival between countries for patients with colon cancer aged 80 years or older. Despite higher prescription of adjuvant chemotherapy, poorer survival outcomes were observed in Belgium. No clear linear pattern between the proportion of chemotherapy and better adjusted relative survival was observed.
Implications for Practice.
With the increasing growth of the older population, clinicians will be treating an increasing number of older patients diagnosed with colon cancer. No clear linear pattern between adjuvant chemotherapy and better adjusted relative survival was observed. Future studies should also include data on surgical quality.
Introduction
Worldwide, one third of all new colon cancer patients are aged 75 years or older [1]. With increasing life expectancy, the number of older colon cancer patients is increasing markedly [2], while evidence from randomized controlled trials for treatment of these patients remains scarce. Older patients may have more comorbidities and frailty that can influence their surgical eligibility [3]. For patients with stage III colon cancer, adjuvant chemotherapy is recommended [4], [5], [6] in order to reduce recurrence rate and improve cancer‐specific survival [7], [8], [9]. For stage II patients, however, the use of adjuvant therapy is still controversial. Especially in the elderly, the benefit of adjuvant chemotherapy is debatable. Currently, chemotherapy with fluoropyrimidines (5‐fluorouracil [5‐FU] or capecitabine) is recommended in older (>70 years) colon cancer patients [4].
The aim of this international comparison is to compare treatment strategies and relative survival (RS) between countries for older patients with colon cancer, using data from population‐based cancer registries across Europe.
Subjects, Materials, and Methods
Study Design
Patients aged 80 years or older diagnosed with colon cancer between 2001 and 2010 were included. Data were collected from six European countries: five national cancer registries and one multicenter cohort from Germany. Three national datasets were obtained from national cancer registries: the Belgian Cancer Registry (BE; 2004–2010), The Netherlands Cancer Registry (NL; 2001–2010), and the Norwegian Cancer Registry (NO; 2001–2010), supplemented with data from the Norwegian Colorectal Cancer Registry (2007–2010) [10]. Denmark (DK) and Sweden (SE) provided data from national clinical quality databases: the Danish Colorectal Cancer Group database (2001–2010) and the Swedish Colorectal Cancer Registry (2007–2010). National datasets (BE, DK, NL, NO, and SE) were included and merged. The cohort ended in June 2014. Data from Germany were not from a national dataset and included only patients who had undergone surgery, and follow‐up data (2001–2004, 2008–2010) were not fully available. Data from Germany were therefore not included in the main analyses but are shown in supplemental online Table 1. Because 5‐year survival for colon cancer has improved over time [11], [12], two time periods according to the different periods for including patients were analyzed: 2001–2006 and 2007–2010. Furthermore, the RS over time, according to country, stage, and period, is shown in supplemental online Table 2.
Quality of the data in terms of completeness (>95% of cancer patients in the population are registered) and accuracy (data on individual cancer patients must be correct) was guaranteed by the cancer registries. Only primary colonic cancers were included. Of patients with metachronous colon cancer, only data of the tumor with highest stage were included for analyses. The pathological tumor stage was provided by the cancer registries, defined using the TNM Classification of Malignant Tumors for colon cancer, 5th, 6th, or 7th edition [13], [14]. In case pathological stage was missing, clinical tumor stage was used. Vital status and date of last follow‐up were established either directly from the patient's medical records or through linkage of cancer registry data with municipal population registries for all countries. Death Certificate Only patients were excluded from the analyses. Follow‐up time for vital status was calculated from date of diagnosis until death, from merge with Municipal Records Database or until end of follow‐up (censored). Patients were excluded from survival analyses if follow‐up data were unavailable or vital status was missing.
Treatment and Outcome
The main outcome was RS expressed as relative excess risk (RER) for each country, compared with a reference category. The secondary outcome was the proportion of given chemotherapy after surgery. The definition of surgery was not uniformly defined among participating countries. In this international comparison, surgical treatment was defined as surgical removal of the tumor, irrespective of curative or palliative intent. Colon resections as well as local removal of the tumor were included. Laparotomy without resection and defunctioning procedures only were not registered as surgical treatment. The proportion of patients receiving surgical treatment according to stage was compared. Adjuvant chemotherapy was defined as the proportion of patients with stage I–III colon cancer who received chemotherapy after surgical treatment. For patients with stage IV colon cancer, chemotherapy is most likely given with palliative intent. Stage IV colon cancer encompasses various clinical conditions and differences in prognosis according to the number and location of metastatic organs. To enhance the uniformity of the data of this group of patients, we chose to determine the proportion of administered chemotherapy in patients who underwent resection of the primary tumor. Data on surgery were complete for all patients. Data on chemotherapy were missing for 0.9% and, in contrast to surgical data, of lower quality for some countries. For example, in Norway, data are based on clinical notifications of planned treatment and not the actual received treatment. The trend of chemotherapy use in different countries was compared with the trend in adjusted RS. In the survival analyses, all patients were included, regardless of treatment modality.
Statistical Analysis
All analyses were stratified for stage of disease. Logistic regression was used to compare the proportion of surgery and chemotherapy between countries, with adjustment for sex, age, and incidence year. For each country, 5‐year RS was calculated as the ratio of the survival observed among the patients diagnosed with colon cancer and the survival that would have been expected based on the corresponding general population, adjusted for age, sex, and year (Ederer II method) [15]. National life tables of each specific country were used to estimate expected survival. To quantify the relative cancer‐related excess mortality between the countries, RER of death was used [16]. RER for countries was estimated using a multivariable generalized linear model with a Poisson distribution, based on collapsed relative survival data, using exact survival times, adjusted for age, sex, and year of diagnosis. The country with the highest proportion of chemotherapy was used as reference category. A higher RER indicates a lower RS. A two‐sided p value <.05 was considered as statistically significant. All analyses were performed in STATA/SE 12.0 (StataCorp, College Station, TX).
Results
Patients and Tumor Characteristics
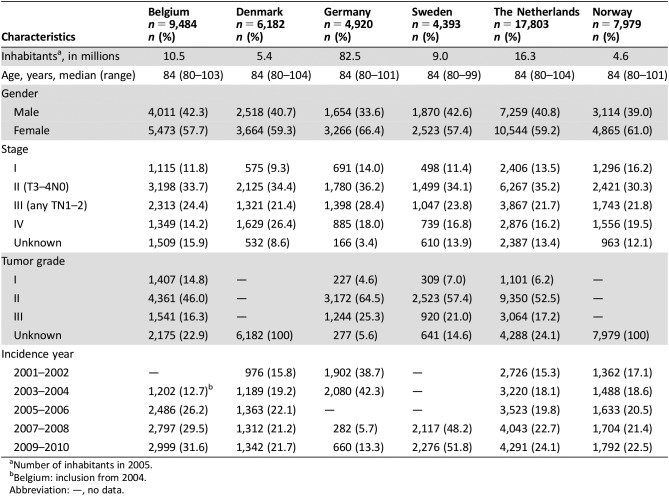
In total, 50,761 patients with colon cancer aged 80 years and older, diagnosed between 2001 and 2010, were included. The numbers of inhabitants per country are described in Table 1. In the cancer registries, follow‐up for vital status was complete for 99.9%. Median follow‐up was 1.6 years (interquartile range [IQR] 0.3–3.8 years). Median follow‐up for patients alive at the end of follow‐up was 3.9 years (IQR 2.5–5.8 years). No differences in stage (p = .39) and grade (p = .40) were observed in patients with known and unknown follow‐up status. Median age was 84 years within every country (range 80–104 years). In Denmark, a lower proportion of stage I (9.3% in DK vs. 16.2% in NO) and a higher proportion of stage IV (26.4% in DK vs. 14.2% in BE) were found (Table 1).
Table 1. Patient and tumor characteristics for patients aged 80 years and older diagnosed with colon cancer (2001–2010), according to country.
Number of inhabitants in 2005.
Belgium: inclusion from 2004.
Abbreviation: —, no data.
Treatment Comparison
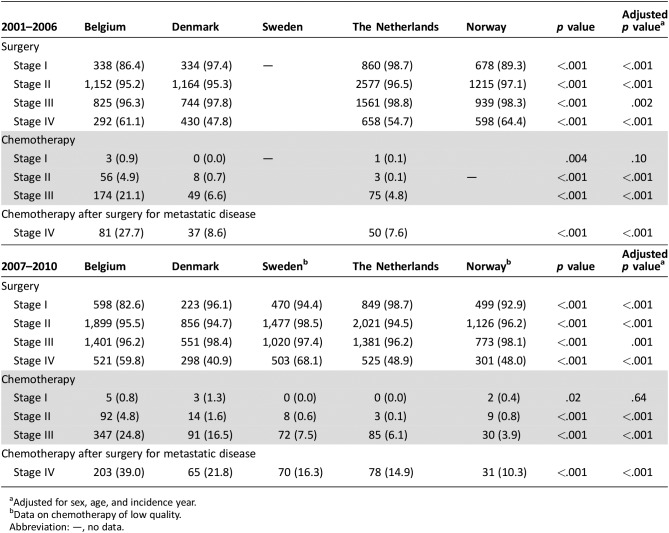
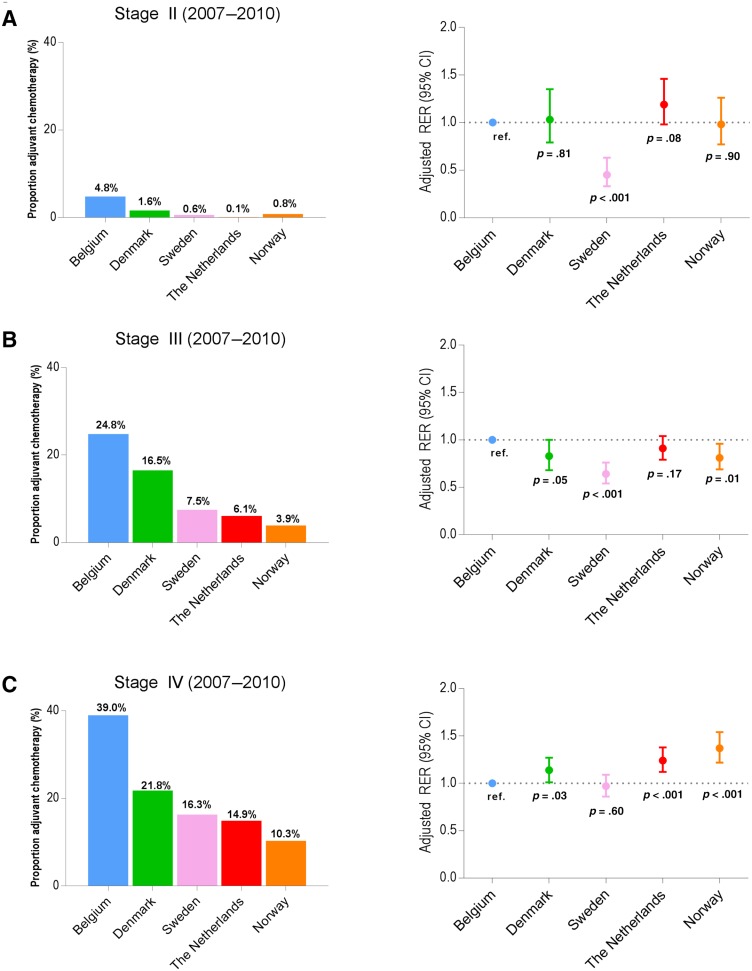
As shown in Table 2, the proportion of patients with stage I colon cancer receiving surgical treatment differed substantially between countries. It was lowest in Belgium (2001–2006: 86.4% and 2007–2010: 82.6%) and highest in The Netherlands (2001–2010: 98.7%). Only a minority of patients were treated with local excision (0.2%–2.8%). The proportion of patients with stage II and III colon cancer undergoing surgery exceeded 94% within every country. Between 2007 and 2010, surgical treatment for stage IV was most pronounced in Belgium (59.8%) and Sweden (68.1%) compared with that in Denmark (40.9%). The proportion of elderly patients who received adjuvant chemotherapy for stage III colon cancer varied between 24.8% (BE) and 3.9% (NO) between 2007 and 2010. For patients with stage IV colon cancer, chemotherapy administration after surgical treatment varied between 39.0% (BE) and 10.3% (NO).
Table 2. Treatment strategies according to stage and period for patients aged 80 years and older diagnosed with colon cancer (2001–2010).
Adjusted for sex, age, and incidence year.
Data on chemotherapy of low quality.
Abbreviation: —, no data.
Survival
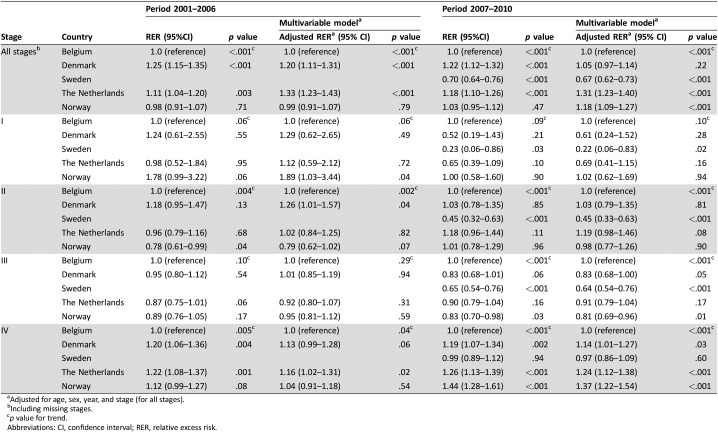
For all countries, decline in RS was most pronounced in the first year (supplemental online Figs. 1, 2). RS increased between 2001–2006 and 2007–2010 for The Netherlands, Denmark, and Norway, but not for Belgium (between 2004–2006 and 2007–2010; supplemental online Table 1). As shown in Table 3, for stage I, a significantly higher RER (lower RS) was observed in Norway compared with Belgium between 2001 and 2006 (adjusted RER 1.89, 95% CI: 1.03–3.44, p = .04). For stage II, between 2001 and 2006, Denmark had higher RER compared with Belgium (adjusted RER 1.26, 95% CI: 1.01–1.57, p = .04). Between 2007 and 2010, these differences were no longer significant for Norway (stage I) and Denmark (stage II). For patients with stage III disease, a significantly better adjusted RS was observed in Sweden (adjusted RER 0.64, 95% CI: 0.54–0.76, p < .001) and Norway (adjusted RER 0.81, 95% CI: 0.69–0.96, p = .01) compared with Belgium between 2007 and 2010. Denmark, The Netherlands, and Norway showed higher RER compared with Belgium for stage IV disease between 2007 and 2010.
Table 3. (Adjusted) RERs of death for patients aged 80 years and older with colon cancer (2001–2010).
Adjusted for age, sex, year, and stage (for all stages).
Including missing stages.
p value for trend.
Abbreviations: CI, confidence interval; RER, relative excess risk.
Survival in Relation to Treatment
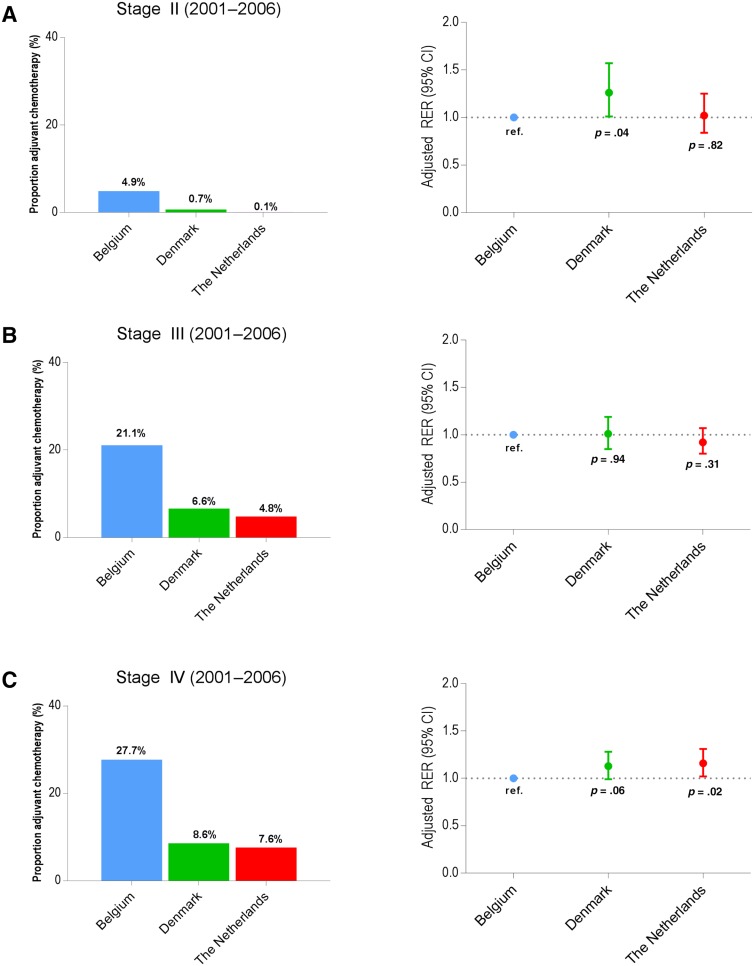
Figures 1 and 2 show the proportion of patients receiving chemotherapy and the adjusted RERs by country. The proportion of administered chemotherapy is determined in patients who underwent resection of the primary tumor; RS is calculated for the country. For stage III, between 2001 and 2006, trends in the proportions of chemotherapy administered in Belgium, Denmark, and The Netherlands were not reflected in significant survival differences between the countries (Fig. 1B). As shown in Figure 2B, representing stage III between 2007 and 2010, patients in Sweden and Norway received less adjuvant chemotherapy compared with Belgium but had a significantly higher RS. For patients with stage IV colon cancer between 2007 and 2010 (Fig. 2C), low proportion of patients receiving chemotherapy seemed to be associated with a significantly lower RS in Denmark, The Netherlands, and Norway.
Figure 1.
Proportion of patients receiving chemotherapy after surgery and adjusted RERs of death for patients 80 years and older diagnosed with stage II colon cancer (A), stage III colon cancer (B), and stage IV colon cancer (C), according to country (2001–2006).
Figure 2.
Proportion of patients receiving adjuvant chemotherapy and adjusted RERs of death for patients 80 years and older diagnosed with stage II colon cancer (A), stage III colon cancer (B), and stage IV colon cancer (C), according to country (2007–2010).
Discussion
This international population‐based comparison shows substantial variation in treatment within five European countries for patients with colon cancer aged 80 years or older, diagnosed between 2001 and 2010. Variation was most pronounced in adjuvant chemotherapy administration. Substantial variation in survival was also observed. No clear pattern between the proportion of chemotherapy and adjusted RS was found.
Patients and Tumor Characteristics
Stage distribution was comparable across participating countries, except for Denmark, where a lower proportion of stage I and a higher proportion of stage IV was found. In countries other than Denmark, there was a higher proportion of unknown stage that might include a high proportion of patients with metastatic disease. Secondly, it is noteworthy that the number of patients diagnosed with colon cancer aged 80 years and older among participating countries in the current study is not always in accordance with the ratio of the number of inhabitants in each country. Possible explanations might be differences in population structure, differences in incidence rates in the older community, or possible differences in early diagnostics.
Treatment Comparison
A notable finding in this study is that almost all patients with stage II and III disease underwent surgical treatment. Only surgical treatment for stage I was performed less often in Belgium and Norway compared with other countries (DK, NL, and SE >94%). On the contrary, for stage IV, surgical treatment was performed more often in Belgium and Sweden (≥60%) compared with other countries. The proportion of older patients treated with adjuvant chemotherapy was highest in Belgium and lowest in The Netherlands and Norway across all stages. This substantial variation cannot be explained by differences between the national guidelines of the participating countries regarding adjuvant chemotherapy, as there were only minor differences in these national guidelines. Only in The Netherlands, (neo)adjuvant chemotherapy was not indicated in the case of resectable metastases (guidelines available on request). The most common reasons for withholding adjuvant chemotherapy in older patients appear to be a combination of high age, comorbidities, and poor performance status or refusal by the patient or family [17].
Adjuvant chemotherapy after resection of the primary tumor reduces the absolute risk of death by 3%–5% in stage II with single‐agent FU and 15%–20% in stage III with FU + oxaliplatin combination [4]. However, frail elderly patients are often excluded from trials. In the MOSAIC trial (FU/leucovorin ± oxaliplatin in stage II and III colon cancer), eligible patients were 18–75 years of age [18]. Subgroup analyses of data from the MOSAIC trial, published in 2012, showed no significant benefit (overall survival and disease‐free survival) for the addition of oxaliplatin to FU as adjuvant treatment for both stage II and older patients (70–75 years) [19]. Single‐agent FU is therefore recommended as the treatment of choice for older patients with high‐risk stage II and stage III patients [4].
Survival
Between 2007 and 2010, for all stages combined, including missing stage, 5‐year RS was lowest in Belgium (52.2%) and highest in Sweden (65.8%; supplemental online Table 1). As shown by EUROCARE‐5, the overall 5‐year RS (all stages) for patients with colon cancer in Europe was 57% but decreased to 49.3% in patients aged 75 years and older [12]. For patients with colon cancer aged 80 years or older, there are no recent published data on variation in survival between countries. Direct comparison with EUROCARE is not possible because these international comparisons included all ages and do not provide further information on stage of disease and given treatments.
Survival in Relation to Treatment
In all countries, almost all patients with stage II and III colon cancer underwent surgical treatment. Unfortunately, no data are available on the intent of surgery and surgical quality. No clear linear pattern was observed between a higher proportion of adjuvant chemotherapy and better adjusted RS. Remarkably, for patients with stage II and III colon cancer in Sweden, significant differences in adjusted RERs are observed, indicating better survival with less chemotherapy. Therefore, this survival difference cannot be explained by beneficial effects of adjuvant chemotherapy, indicating a major role for quality of care. This might be explained by the focus on improved colon cancer treatment on a national level since 2004–2005 in Sweden, including better preoperative work‐up, improved surgery with the concept of complete mesocolic excision, less acute resections, and better pathology assessment [20]. Several other explanations for survival differences have previously been suggested. These include demographic differences, differences in lifestyle, stage at diagnosis, and health care systems, and differences in access to or use of effective treatment options [21]. However, one should consider the fact that data on chemotherapy for some countries are of lower quality and compliance is unknown. Differences in survival can also be caused by differences other than treatment, such as peri‐operative care and selection of patients. Only for patients with stage IV disease between 2007 and 2010, Denmark, The Netherlands, and Norway described less chemotherapy after surgical treatment and showed lower adjusted RS compared with Belgium. However, not the administration of chemotherapy but the higher proportion of surgical treatment for stage IV colon cancer in Belgium and Sweden may explain the higher survival rates in these two countries.
Strengths and Limitations
This study is unique in comparing both treatment and RS between European countries for patients aged 80 years or older with colon cancer. This population‐based study offers insight in the use of surgery and adjuvant chemotherapy among those elderly patients who are often excluded in clinical trials. Observational data from cancer registries are highly representative for the older population because there is no selection for inclusion. Furthermore, major strengths are the large number of colon cancer cases included and representation of different countries in Western and Northern Europe.
This study has some limitations. Most importantly, there might be unknown differences in data registration between the countries. Also, residual confounding by unmeasured factors cannot be excluded, as no information was available on, among others, vascular or lymphatic invasion, surgical quality, postoperative complications, chemotherapy compliance, and comorbidity. Finally, data for stage as well as emergency surgery were incomplete and may have a substantial effect on outcomes.
Clinical Implications
With the increasing growth of the older population, clinicians will be treating an increasing number of older patients diagnosed with colon cancer. Because no clear linear pattern between adjuvant chemotherapy and better adjusted RS was observed in the countries in this study, further detailed studies are necessary to improve evidence for the indication of chemotherapy in the oldest population. Future studies should also include data on surgical quality. However, quantifying surgical quality remains challenging.
Conclusion
The present international comparison from the EURECCA colon cancer group shows substantial variation in treatment for patients diagnosed with colon cancer and aged 80 years or older. Especially in higher stages of disease, variation in adjuvant chemotherapy administration was pronounced. However, no clear pattern between the proportion of chemotherapy and better adjusted RS was observed.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
The authors would like to thank A.J. Breugom and M. Kiderlen for collecting and analysing German data, H. Ortiz and V. Valentini for critical review of the study proposal, H. Rutten, V. Lemmens, and L. Pahlman for discussing the proposal and first results and P.G. Boelens for discussing the proposal and paper.
Author Contributions
Conception/design: Nina C.A. Vermeer, Cornelis J.H. van de Velde, Esther Bastiaannet
Provision of study material or patients: Lene H. Iversen, Elizabeth van Eycken, Marianne G. Guren, Pawel Mroczkowski, Anna Martling, Robert Johansson, Tamara Vandendael, Arne Wibe, Bjorn Moller, Hans Lippert, Esther Bastiaannet
Collection and/or assembly of data: Nina C.A. Vermeer, Lene H. Iversen, Elizabeth van Eycken, Marianne G. Guren, Pawel Mroczkowski, Anna Martling, Robert Johansson, Tamara Vandendael, Arne Wibe, Bjorn Moller, Hans Lippert, Esther Bastiaannet
Data analysis and interpretation: Nina C.A. Vermeer, Yvette H.M. Claassen, Marloes G.M. Derks, Lene H. Iversen, Elizabeth van Eycken, Marianne G. Guren, Anna Martling, Robert Johansson, Tamara Vandendael, Arne Wibe, Bjorn Moller, Hans Lippert, Esther Bastiaannet
Manuscript writing: Nina C.A. Vermeer, Yvette H.M. Claassen, Marloes G.M. Derks, Lene H. Iversen, Elizabeth van Eycken, Marianne G. Guren, Anna Martling, Johanneke E.A. Portielje, Gerrit Jan Liefers, Koen C.M.J. Peeters, Cornelis J.H. van de Velde, Esther Bastiaannet
Final approval of manuscript: Nina C.A. Vermeer, Yvette H.M. Claassen, Marloes G.M. Derks, Lene H. Iversen, Elizabeth van Eycken, Marianne G. Guren, Anna Martling, Arne Wibe, Johanneke E.A. Portielje, Gerrit Jan Liefers, Koen C.M.J. Peeters, Cornelis J.H. van de Velde, Esther Bastiaannet
Disclosures
The authors indicated no financial relationships.
References
- 1.Institute NC . SEER Stat Fact Sheets: Colon and Rectum Cancer. Available at https://seer.cancer.gov/statfacts/html/colorect.html. Accessed August 2016.
- 2. Pallis AG, Papamichael D, Audisio R et al. EORTC Elderly Task Force experts' opinion for the treatment of colon cancer in older patients. Cancer Treat Rev 2010;36:83–90. [DOI] [PubMed] [Google Scholar]
- 3. Dekker JW, van den Broek CB, Bastiaannet E et al. Importance of the first postoperative year in the prognosis of elderly colorectal cancer patients. Ann Surg Oncol 2011;18:1533–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schmoll HJ, Van Cutsem E, Stein A et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer: A personalized approach to clinical decision making. Ann Oncol 2012;23:2479–2516. [DOI] [PubMed] [Google Scholar]
- 5. Schmoll HJ, Twelves C, Sun W et al. Effect of adjuvant capecitabine or fluorouracil, with or without oxaliplatin, on survival outcomes in stage III colon cancer and the effect of oxaliplatin on post‐relapse survival: A pooled analysis of individual patient data from four randomised controlled trials. Lancet Oncol 2014;15:1481–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Breugom AJ, Bastiaannet E, Boelens PG et al. Adjuvant chemotherapy and relative survival of patients with stage II colon cancer ‐ A EURECCA international comparison between the Netherlands, Denmark, Sweden, England, Ireland, Belgium, and Lithuania. Eur J Cancer 2016;63:110–117. [DOI] [PubMed] [Google Scholar]
- 7. Moertel CG. Chemotherapy for colorectal cancer. N Engl J Med 1994;330:1136–1142. [DOI] [PubMed] [Google Scholar]
- 8. O'Connell MJ, Mailliard JA, Kahn MJ et al. Controlled trial of fluorouracil and low‐dose leucovorin given for 6 months as postoperative adjuvant therapy for colon cancer. J Clin Oncol 1997;15:246–250. [DOI] [PubMed] [Google Scholar]
- 9.Efficacy of adjuvant fluorouracil and folinic acid in colon cancer . International Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT) investigators. Lancet 1995;345:939–944. [PubMed] [Google Scholar]
- 10. Nedrebo BS, Soreide K, Eriksen MT et al. Survival effect of implementing national treatment strategies for curatively resected colonic and rectal cancer. Br J Surg 2011;98:716–723. [DOI] [PubMed] [Google Scholar]
- 11. Allemani C, Weir HK, Carreira H et al. Global surveillance of cancer survival 1995–2009: Analysis of individual data for 25,676,887 patients from 279 population‐based registries in 67 countries (CONCORD‐2). Lancet 2015;385:977–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Holleczek B, Rossi S, Domenic A et al. On‐going improvement and persistent differences in the survival for patients with colon and rectum cancer across Europe 1999–2007 ‐ Results from the EUROCARE‐5 study. Eur J Cancer 2015;51:2158–2168. [DOI] [PubMed] [Google Scholar]
- 13. Sobin LH, Compton CC. TNM seventh edition: What's new, what's changed: Communication from the International Union Against Cancer and the American Joint Committee on Cancer. Cancer 2010;116:5336–5339. [DOI] [PubMed] [Google Scholar]
- 14. Puppa G, Sonzogni A, Colombari R et al. TNM staging system of colorectal carcinoma: A critical appraisal of challenging issues. Arch Pathol Lab Med 2010;134:837–852. [DOI] [PubMed] [Google Scholar]
- 15. Hakulinen T, Seppa K, Lambert PC. Choosing the relative survival method for cancer survival estimation. Eur J Cancer 2011;47:2202–2210. [DOI] [PubMed] [Google Scholar]
- 16. Suissa S. Relative excess risk: An alternative measure of comparative risk. Am J Epidemiol 1999;150:279–282. [DOI] [PubMed] [Google Scholar]
- 17. Hoeben KW, van Steenbergen LN, van de Wouw AJ et al. Treatment and complications in elderly stage III colon cancer patients in the Netherlands. Ann Oncol 2013;24:974–979. [DOI] [PubMed] [Google Scholar]
- 18. Andre T, Boni C, Navarro M et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009;27:3109–3116. [DOI] [PubMed] [Google Scholar]
- 19. Tournigand C, Andre T, Bonnetain F et al. Adjuvant therapy with fluorouracil and oxaliplatin in stage II and elderly patients (between ages 70 and 75 years) with colon cancer: Subgroup analyses of the Multicenter International Study of Oxaliplatin, Fluorouracil, and Leucovorin in the Adjuvant Treatment of Colon Cancer trial. J Clin Oncol 2012;30:3353–3360. [DOI] [PubMed] [Google Scholar]
- 20. Bernhoff R, Martling A, Sjovall A et al. Improved survival after an educational project on colon cancer management in the county of Stockholm–A population based cohort study. Eur J Surg Oncol 2015;41:1479–1484. [DOI] [PubMed] [Google Scholar]
- 21. Ait Ouakrim D, Pizot C, Boniol M et al. Trends in colorectal cancer mortality in Europe: Retrospective analysis of the WHO mortality database. BMJ 2015;351:h4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.