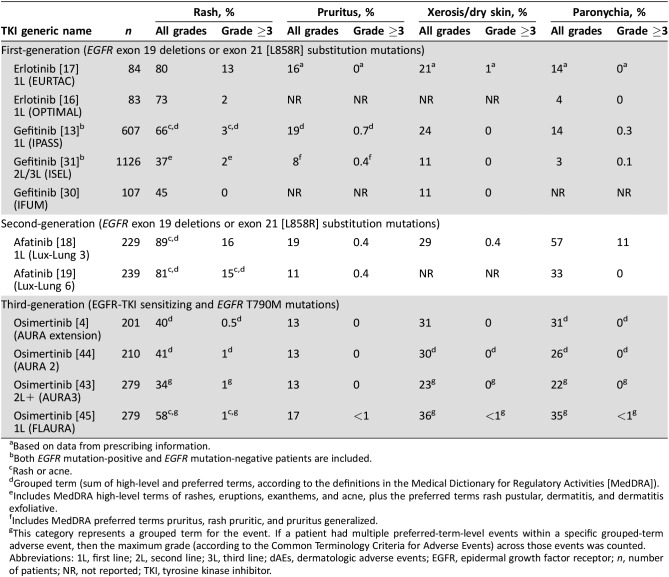
Table 1. Incidence of dermatologic adverse events associated with EGFR‐TKIs approved by the U.S. Food and Drug Administration for the treatment of metastatic EGFR mutation‐positive non‐small cell lung cancer (based on data from pivotal clinical trials).
Based on data from prescribing information.
Both EGFR mutation‐positive and EGFR mutation‐negative patients are included.
Rash or acne.
Grouped term (sum of high‐level and preferred terms, according to the definitions in the Medical Dictionary for Regulatory Activities [MedDRA]).
Includes MedDRA high‐level terms of rashes, eruptions, exanthems, and acne, plus the preferred terms rash pustular, dermatitis, and dermatitis exfoliative.
Includes MedDRA preferred terms pruritus, rash pruritic, and pruritus generalized.
This category represents a grouped term for the event. If a patient had multiple preferred‐term‐level events within a specific grouped‐term adverse event, then the maximum grade (according to the Common Terminology Criteria for Adverse Events) across those events was counted.
Abbreviations: 1L, first line; 2L, second line; 3L, third line; dAEs, dermatologic adverse events; EGFR, epidermal growth factor receptor; n, number of patients; NR, not reported; TKI, tyrosine kinase inhibitor.