Streptococcus zooepidemicus is an emerging and opportunistic zoonotic pathogen which plays an important role in the development of severe and life-threatening diseases and is potentially capable of triggering large glomerulonephritis outbreaks. Between December 2012 and February 2013, 175 cases of glomerulonephritis were confirmed in the town of Monte Santo de Minas, MG, Brazil.
KEYWORDS: group C Streptococcus, milk, ice cream, nephritis, rep-PCR, fingerprint patterns, DiversiLab, SzP protein, MALDI-TOF
ABSTRACT
Streptococcus zooepidemicus is an emerging and opportunistic zoonotic pathogen which plays an important role in the development of severe and life-threatening diseases and is potentially capable of triggering large glomerulonephritis outbreaks. Between December 2012 and February 2013, 175 cases of glomerulonephritis were confirmed in the town of Monte Santo de Minas, MG, Brazil. During the outbreak, 19 isolates of S. zooepidemicus were recovered, 1 from ice cream, 2 from the oropharynx of food handlers, and 16 from patients affected by acute poststreptococcal glomerulonephritis (APSGN). All S. zooepidemicus isolates involved in the outbreak amplified the same sequence of the hypervariable region of the SzP protein (SzPHV5) and presented indistinguishable banding patterns with high similarity (>99%) to each other by the repetitive element sequence-based PCR (rep-PCR) technique. Inspection programs on the milk supply chain should be strengthened and continuously encouraged so that the health of consumers is preserved.
INTRODUCTION
Streptococcus equi subsp. zooepidemicus (Lancefield group C Streptococcus [GCS]) is a common inhabitant of the respiratory and intestinal tracts of horses, which, in special circumstances, is a devastating and fatal pathogen for several animals (1). Humans (2) become infected when they come into contact with colonized or sick animals or when they consume unpasteurized milk or its derived products (3, 4). Infections caused by this microorganism have a wide spectrum of severity. They cause serious invasive diseases (bacteremia, septic arthritis, pneumonia, and meningitis), posing a risk of death for the affected individuals (2, 5), or benign diseases, such as pharyngitis, but which can be followed by an episode of acute poststreptococcal glomerulonephritis (APSGN) (4, 6, 7). APSGN is mediated by the deposition of immune complexes that lodge in the renal glomerulus after 7 to 21 days of primary streptococcal infection. The lesions resulting from this process lead to renal failure, albuminuria, hematuria, hypertension, and edema (8, 9). The disease is rare in industrialized nations; however, in the underprivileged world, new cases of APSGN range between 9.5 and 28.5 per 100,000 individuals per year (10).
APSGN is often triggered by the nephritogenic M types of Streptococcus pyogenes (Lancefield group A Streptococcus [GAS]) (7) or, less frequently, by species of S. zooepidemicus and S. dysgalactiae subsp. equisimilis (Lancefield groups C and G) (3, 11, 12).
S. zooepidemicus has a protein called SzP on its cell surface, which exhibits antiphagocytic (M-like) properties similar to those found in GAS (13). The SzP protein varies the composition of amino acids within the hypervariable (HV) region and the amount of tetrapeptides (7 to 12 proline-glutamic acid-proline-lysines [PEPK]) of proline-glutamic acid-proline-lysine in the COOH-terminal region, determining 5 different HV types (HV1 to HV5) (14). The hypervariable region of SzP can be used to genetically differentiate strains within the subspecies (5).
Studies have reported the use of repetitive element sequence-based PCR (rep-PCR) for the molecular typing of microorganisms. This method uses oligonucleotides complementary to repetitive, highly conserved DNA sequences present at numerous copies in the bacterial genome. It enables genotypic characterization, clone differentiation, and clone dispersion in the community (15, 16).
Recently we described, in a case-control study report (17), a large outbreak of APSGN caused by S. zooepidemicus in the small town of Monte Santo de Minas (population of 21,234 inhabitants), which is predominantly urban (77.4%) and located in the south of Minas Gerais State. The cases were associated with the consumption of milk and its derived products. Among the affected patients, 42 (24.0%) were hospitalized, and the course of the disease in 4 individuals progressed to acute renal failure and necessitated hemodialysis. No deaths were recorded.
The objective of this study was 2-fold. First, it aimed to identify S. zooepidemicus strains collected from food handlers' oropharynx secretions and from milk, cheese, and ice cream samples during the glomerulonephritis outbreak in Monte Santo de Minas. Second, it attempted to genetically characterize the S. zooepidemicus strains isolated from the affected patients and from other sources during the outbreak by sequencing the hypervariable region of the SzP protein and the DNA fingerprint patterns with DiversiLab software.
MATERIALS AND METHODS
Study outline.
Between December 25, 2012, and February 18, 2013, 417 suspected cases of APSGN were reported, and 175 (42.0%) of them were confirmed by the Municipal Health Department of Monte Santo de Minas, MG, Brazil. The cases were confirmed with the following criteria: a patient resident in the city of Monte Santos de Minas diagnosed with pharyngitis, with or without isolation of Streptococcus zooepidemicus, (i) followed by hematuria, with or without fever, myalgia, and headache, or (ii) followed by acute nephritis, characterized by the presence of hematuria, edema, oliguria, and arterial hypertension. Out of these patients, 118 were female (67.4%), and their median age was 36 years. In the adjusted analysis of the case-control study (17), the consumption of ice cream (odds ratio [OR], 9.67; 95% confidence interval [CI], 2.08 to 44.89) and milk in plastic packaging (OR, 4.04; 95% CI, 1.43 to 11.47), provided by one of the town dairies, was associated with the outbreak.
To isolate the etiological agent identified in the outbreak, we collected 104 cow milk samples from several rural properties, 1 sample from the mechanical milking machine, 3 samples from the storage tank, 25 samples of locally produced dairy-based food products (cheese and ice cream), 11 samples of oropharyngeal secretions from food handlers (who worked in 5 different ice cream parlors, 1 bakery, and 1 dairy shop, located in different parts of the town), and 36 samples of oropharyngeal secretions from patients who developed pharyngitis and subsequent APSGN.
The samples were submitted for testing to the Laboratory of Microbiology of Foods, Ezequiel Dias Foundation, Central Laboratory of Minas Gerais (Lacen, MG, Brazil). The samples of raw cow milk and isolates of group C beta-hemolytic streptococci identified in an ice cream sample, 2 handlers, and 16 patients were tested at the Public Health Laboratory of the State of Paraná–Lacen/PR, Collaborating Center of the Ministry of Health, MS, Brazil, for the confirmation of species/subspecies and molecular characterization.
Microbiologic Isolation and Identification.
Samples of raw milk were analyzed as previously described (4) with the following modifications: (i) direct inoculation onto the surface of blood agar plates (BAPs), (ii) 1-ml inoculation of the sample in 10 ml of Todd-Hewitt broth (THB; BBL Microbiology Systems, Cockeysville, MD, USA), and (iii) 1-ml inoculation of the sample in 10 ml of THB supplemented with nalidixic acid (15 mg/liter) and colistin (10 mg/liter). The cheese and ice cream samples were serially diluted in 0.1% buffered peptone water and then striated onto the BAPs. Samples of oropharyngeal secretions from the patients and handlers and from the milking machine teat cups were directly streaked onto the BAPs.
The GCS isolates were presumptively identified based on their phenotypic characteristics on BAPs after incubation at 36°C ± 1°C under an aerobic atmosphere. The beta-hemolytic, catalase-negative, Gram-positive cocci arranged in chains and latex agglutination test group C-specific antisera (streptococcal grouping KitR, Oxoid, Basingstoke, England) were identified by the biochemical tests deamination of arginine, hydrolysis of starch, and production of acid in sorbitol, trehalose, lactose, and ribose (18, 19). Matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS; Vitek MS, bioMérieux) was also employed to confirm the identity of the strains involved in the outbreak (20).
PCR and sequence analysis.
The SzP gene was sequenced, and the presumed protein sequence was determined for each isolate. PCR and DNA sequencing were performed as previously described (21), with PCR and sequencing primers cf1 (GATAATTAGGAGACATCATGTCTAGATA), cf2 (GGCTAGCTTCAGTATCGGCAGCCTTGT), cr1 (AAGCTTTACCACTGGGGTAT), and cr2 (GCAAGAGCTGCCGCGGTGAAGAATGGAT) derived from the sequence with GenBank accession number U04620 (bases 181 to 208, 274 to 300, 1362 to 1383, and 1276 to 1303, respectively) (22).
DiversiLab semiautomated rep-PCR.
Genomic DNA was extracted from a 10-μl loopful of S. zooepidemicus colonies using the UltraClean microbial DNA isolation kit (Mo Bio Laboratories, Inc., Solana Beach, CA). PCR was performed using the DiversiLab Streptococcus kit (bioMérieux). PCR products were separated in a microfluidics DNA chip device (bioMérieux) in the Agilent 2100 Bioanalyzer (Agilent Technologies, Inc., Palo Alto, CA) according to the manufacturer's recommendations. The relatedness was determined by cluster analysis and guidelines provided by the manufacturer. Isolates were categorized as indistinguishable, similar, or different. In general, “different” was defined as ≤95% similarity and 2 or more band differences, “similar” was defined as ≥95% similarity and 1 band difference, and “indistinguishable” was defined as ≥95% similarity and no band differences (16).
Statistics.
The DNA fingerprint patterns were analyzed with the DiversiLab software version 1.2.66 (DiversiLab, bioMérieux, France), using the Pearson correlation coefficient to determine distance matrices and unweighted pair group method with arithmetic mean (UPGMA) to create dendrograms, scatter plots, and electropherograms for data interpretation. Categorical variables are shown as frequencies and percentages.
Accession number(s).
The szm gene nucleotide sequences of the 19 isolates of S. zooepidemicus identified during the outbreak (Fig. 1) have been deposited in GenBank under accession numbers MH614394, MH614395, MH614396, MH614397, MH614398, MH614399, MH614400, MH614401, MH614402, MH614403, MH614404, MH614405, MH614406, MH614407, MH614408, MH614409, MH614410, MH614411, and MH614412.
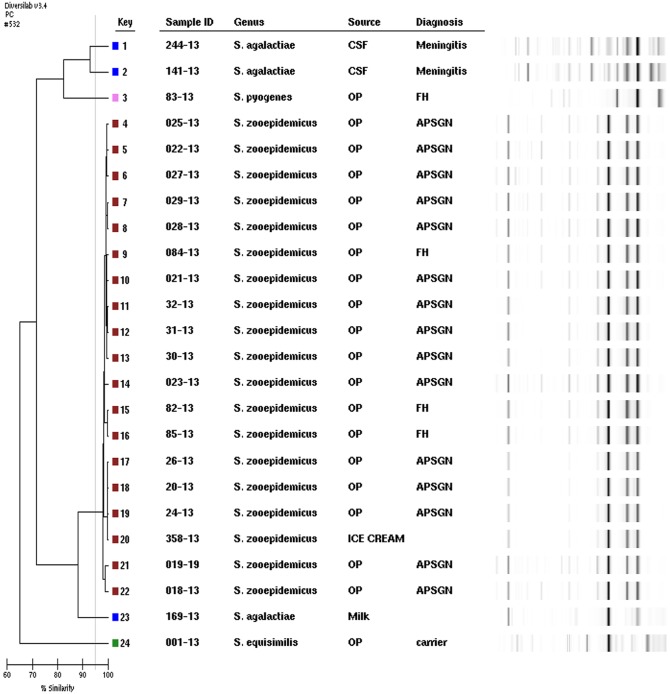
FIG 1.
Rep-PCR dendrogram and simulated electrophoresis. Strains are numbered 1 to 24, and fingerprint types (P) are color coded. Internal control, S. agalactiae meningitis isolate corresponding to keys 1 and 2; S. dysgalactiae subsp. equisimilis carrier isolate, key 24; S. zooepidemicus (outbreak strains) corresponding to keys 4 through 22; S. agalactiae isolated from milk, key 23, and S. pyogenes emm type 3 oropharynx of food handler, key 3. OP, oropharynx; FH, food handler.
RESULTS
The isolates of S. zooepidemicus were identified by the presence of beta-hemolysis, group C antigen, hydrolysis of esculin and starch, resistance to bacitracin, negative reactions in CAMP and Voges-Proskauer tests, deamination of arginine, and fermentation of ribose, sorbitol, and trehalose and were confirmed by mass spectrometry (MALDI-TOF MS).
The samples obtained from the milk, storage tank, and milking machine were negative for S. zooepidemicus. However, in 10 (9.6%) of the milk samples, growth of Streptococcus agalactiae (Lancefield group B Streptococcus [GBS]) was identified.
Among the 25 milk-derived food samples (from cheese and ice cream) analyzed, only 1 (4.0%) ice cream sample, collected in one of the APSGN patient's freezer, was positive for S. zooepidemicus.
Among 175 confirmed APSGN patients, 36 oropharyngeal samples were analyzed, and 16 (44.4%) had a positive culture test. Many culture-negative patients had previously been treated with antimicrobials. Out of the 11 oropharyngeal samples from food handlers examined, 2 were positive for S. zooepidemicus (1 was from an asymptomatic carrier and 1 from a carrier presenting pharyngitis symptoms [fever, swallowing pain, hyperemia, edema, and a pus-filled spot on one of the tonsils] who developed APSGN), 8 were negative for this pathogen, and 1 was identified as carrying GAS emm type 3. All patients involved in the outbreak were treated with 500 mg amoxicillin every 8 h for 10 days. After the end of the antibiotic therapy, a new collection of oropharyngeal secretion samples was obtained. All cultures were negative for S. zooepidemicus.
The chromosomal templates from the 19 outbreak/2012–2013/Monte Santo-MG-Brazil isolates of S. zooepidemicus amplified the szp gene as expected, and all PCR products shared sequence identity across their entire length (1,128-bp structural gene plus 19 bp of upstream sequence). These isolates were compared with strains of szp HV5-5058-APSGN/Nova Serrana-MG, Brazil/1998 (22) and revealed 100% identity with the 183 bases of the HV region.
Highly conserved regions of bacterial DNA of the 19 isolates of S. zooepidemicus were amplified to create a matrix of genetic proximity. Molecular typing (rep-PCR) produced a unique electrophoretic pattern (G1) of high genetic similarity (≥99%), and no different band was identified among the S. zooepidemicus isolates from different sites, oropharynx of patients, food, and ice cream handlers (Fig. 1).
DISCUSSION
Outbreaks induced by emergent pathogens represent a health risk factor for a large number of individuals if quality controls in the production of milk and in the chain of milk-derived subproducts are not preserved. S. zooepidemicus is an emerging and opportunistic zoonotic pathogen associated with severe and life-threatening diseases and is capable of triggering large glomerulonephritis outbreaks. It causes diseases in humans through contact with infected animals (23–26) and by the consumption of milk and dairy products (3, 4).
This study discussed genetically isolated S. zooepidemicus associated with a large outbreak of pharyngitis and APSGN in Monte Santo de Minas, MG, Brazil. The microorganism was identified in the oropharynx of APSGN patients, food handlers, and ice cream samples. The same sequence of the hypervariable region of the SzP protein was amplified in all isolates involved in the outbreak, and these isolates were classified as SzPHV5. The isolates were categorized as clonal due to their indistinguishable banding patterns with high similarity (>99%) by rep-PCR, characterizing the genetic relationship between them and confirming the outbreak.
Previous studies reported a milk-borne outbreak with 85 cases of sore throat and, later, APSGN in the town of Piatra Neamt, Romania (1968), where the S. zooepidemicus szpHV3 gene was identified in lymph node biopsy specimens and pharyngeal exudate of patients, in carriers, and in cow milk samples (22–27). Fifteen years later, another outbreak attributed to S. zooepidemicus HV5 took place in North Yorkshire, England (1983). Three cases of nephritis after mild upper respiratory tract infection were identified in 5 members of a family running a small dairy farm; of the 12 people hospitalized, 8 died (3). These outbreaks implicated non-group A streptococci in the etiology of APSGN.
In Brazil, S. zooepidemicus szpHV5 was responsible for a large outbreak of APSGN (1997 to 1998) in the city of Nova Serrana, Minas Gerais, Brazil. At that time, 133 cases of APSGN were confirmed, 3 patients died, APSGN in 7 patients progressed to acute renal failure and necessitated dialysis, and 96 patients were hospitalized. During the outbreak, 4 isolates were recovered among the patients. No isolate was recovered in the analyzed milk or cheese samples (4). The sequence described in the current study shared sequence identity with SS1215 APSGN/England/1992 (3) and szp5058-APSGN/Nova Serrana-MG, Brazil/1998 (22) but not with szp1028 APSGN/Romania 1968 (22–27).
The szp gene shares structural and functional similarity with the GAS M protein, mainly with the prototype of the subfamily E emm types (2, 4, 49, 60, 61, and 63). Moreover, it does not have the amino-terminal regions (A and B) repeated from the N-terminal region, and it does not have the C region repeated from the carboxy-terminal region (found in all GAS emm types) (14, 28). Considering that both species are capable of triggering glomerulonephritis and that several studies have associated GAS protein M with the development of APSGN, we can infer that rheumatoid epitopes are located in the carboxy-terminal D region.
Genotyping is a valuable tool for epidemiological surveillance of microorganisms, leading to an efficient investigation of microbial diversity, microevolutionary shifts, and transmission tracking. The diversity of the SzP proteins HV1 through HV5 may establish the clonal characteristics of the infections of the respiratory tract of horses (29) and may also genetically characterize strains associated with outbreaks in humans (3, 22, 27).
It was not yet possible to observe any relationship between a type of SzP and a specific clinical manifestation in horses (14). However, S. zooepidemicus SzPHV5 is likely to present superior nephritogenic properties compared with those of the other SZP protein types.
The SzP (M-like) protein is capable of inducing a specific protective immune response against infection by S. zooepidemicus. Specific antibodies targeting SzP proteins were associated with generalized diffuse glomerulonephritis in horses (6) and were also found in the serum of patients recovering from APSGN. Serum from patients in their convalescent phase revealed the presence of antibodies highly reactive against the antigens of the isolated strains, associating them with the cause of the PSGN outbreak (22). APSGN is widely believed to be caused when antibody-antigen immune complexes, lodged in the kidney glomerulus, trigger proinflammatory immunologic processes and produce organ injury. Many extracellular streptococcal products have been causally implicated, including streptokinase, streptococcal pyrogenic exotoxin B (SpeB, an extracellular cysteine protease), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and others. However, the absence of cysteine proteinase (SpeB) in the strain szp5058-APSGN/Nova Serrana-MG, Brazil/1998 contradicted the belief that cysteine proteinase could induce PSGN (30).
At least three outbreaks have now been reported in the world. The isolation of S. zooepidemicus in an ice cream sample confirmed the case-control study carried out at the time of the outbreak (2012 to 2013) and associated ice cream with the disease transmission chain. It is possible to infer that the epitopes of the innermost portion of the protein M GAS, SzPHV5, and SzPHV3 (D region) may be involved in the development of APSGN, and related studies should be conducted. Stricter controls and supervision in the production of milk and in the dairy by-products chain should be continuously encouraged so that the health of consumers is preserved.
REFERENCES
- 1.Pisoni G, Zadoks RN, Vimercati C, Locatelli C, Zanoni MG, Moroni P. 2009. Epidemiological investigation of Streptococcus equi subspecies zooepidemicus involved in clinical mastitis in dairy goats. J Dairy Sci 92:943–951. doi: 10.3168/jds.2008-1548. [DOI] [PubMed] [Google Scholar]
- 2.Bordes-Benítez A, Sánchez-Oñoro M, Suárez-Bordón P, García-Rojas AJ, Saéz-Nieto JA, González-García A, Alamo-Antúnez I, Sánchez-Maroto A, Bolaños-Rivero M. 2006. Outbreak of Streptococcus equi subsp. zooepidemicus infections on the island of Gran Canaria associated with the consumption of inadequately pasteurized cheese. Eur J Clin Microbiol Infect Dis 25:242–246. [DOI] [PubMed] [Google Scholar]
- 3.Barham M, Thorton TJ, Lange K. Nephritis caused by Streptococcus zooepidemicus (Lancefield group C). 1983. Lancet 30:945–948. [DOI] [PubMed] [Google Scholar]
- 4.Baiter S, Benin A, Pinto SWL, Teixeira LM, Alvim GG, Luna E, Jackson D, LaClaire L, Elliott J, Facklam R, Schuchat A. 2000. Epidemic nephritis in Nova Serrana, Brazil. Lancet 355:1776–1780. doi: 10.1016/S0140-6736(00)02265-0. [DOI] [PubMed] [Google Scholar]
- 5.Pelkonen S, Lindahl SB, Suomala P, Karhukorpi J, Vuorinen S, Koivula I, Väisänen T, Pentikäinen J, Autio T, Tuuminen T. 2013. Transmission of Streptococcus equi subspecies zooepidemicus infection from horses to humans. Emerg Infect Dis 19:1041–1048. doi: 10.3201/eid1907.121365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Divers TJ, Timoney JF, Lewis RM, Smith CA. 1992. Equine glomerulonephritis and renal failure associated with complexes of group-C streptococcal antigen and IgG antibody. Vet Immunol Immunopathol 32:93–102. doi: 10.1016/0165-2427(92)90071-W. [DOI] [PubMed] [Google Scholar]
- 7.Zheng M-H, Jiao Z-Q, Zhang L-J, Yu S-J, Tang G-P, Yan X-M, He L-H, Meng F-L, Zhao F, Zhang M-J, Xiao D, Yang Y-H, Nie W, Zhang J-Z, Wang Z-J. 2009. Genetic analysis of group A Streptococcus isolates recovered during acute glomerulonephritis outbreaks in Guizhou Province of China. J Clin Microbiol 47:715–720. doi: 10.1128/JCM.00747-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nordstrand A, Norgren M, Holm SE. 1999. Pathogenic mechanism of acute post-streptococcal glomerulonephritis. Scand J Infect Dis 31:523–537. doi: 10.1080/00365549950164382. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Iturbe B, Batsford S. 2007. Pathogenesis of poststreptococcal glomerulonephritis a century after Clemens von Pirquet. Kidney Int 71:1094–1104. doi: 10.1038/sj.ki.5002169. [DOI] [PubMed] [Google Scholar]
- 10.Rodríguez-Iturbe B, Musser JM. 2008. The current state of poststreptococcal glomerulonephritis. J Am Soc Nephrol 19:1855–1864. doi: 10.1681/ASN.2008010092. [DOI] [PubMed] [Google Scholar]
- 11.Gnann JW, Gray BM, Griffin FM, Dismuke WE. 1987. Acute glomerulonephritis following group G streptococcal infection. J Infect Dis 156:411–412. doi: 10.1093/infdis/156.2.411. [DOI] [PubMed] [Google Scholar]
- 12.Tewodros W, Muhe L, Daniel E, Schalén C, Kronvall G. 1992. A one-year study of streptococcal infections and their complications among Ethiopian children. Epidemiol Infect 109:211–225. doi: 10.1017/S0950268800050172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Timoney JF, Walker J, Zhou M, andDing J. 1995. Cloning and sequence analysis of a protective M-like protein gene from Streptococcus equi subsp. zooepidemicus. Infect Immun 63:1440–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walker JA, Timoney JF. 1998. Molecular basis of variation in protective SzP proteins of Streptococcus zooepidemicus. Am J Vet Res 59:1129–1133. [PubMed] [Google Scholar]
- 15.Shutt CK, Pounder JI, Page SR, Schaecher BJ, Woods GL. 2005. Clinical evaluation of the DiversiLab microbial typing system using repetitive-sequence-based PCR for characterization of Staphylococcus aureus strains. J Clin Microbiol 43:1187–1192. doi: 10.1128/JCM.43.3.1187-1192.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torres RSLA, Santos TZ, Torres RAA, Pereira VVG, Fávero LAF, Filho ORM, Penkal ML, Araujo LS. 2015. Resurgence of pertussis at the age of vaccination: clinical, epidemiological, and molecular aspects. J Pediatr (Rio J) 91:333–338. doi: 10.1016/j.jped.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Soares PA, Duarte HHP, Oliveira JV, Faúla LL, Torres RSLA, Penkal ML, Guimarães MDC. 2017. Outbreak of nephritis by Streptococcus equi subspecies zooepidemicus: case-control study in the municipality of Monte Santo de Minas, Minas Gerais, Brazil, 2013. Epidemiol Serv Saude 26:405–412. doi: 10.5123/S1679-49742017000200018. [DOI] [PubMed] [Google Scholar]
- 18.Facklam R. 2002. What happened to the streptococci: overview of taxonomic and nomenclature changes. Clin Microbiol Rev 15:613–630. doi: 10.1128/CMR.15.4.613-630.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torres RSLA, Paula CC, Pilonetto M, Fontana CK, Minozzo JC, Torres RA. 2007. An outbreak of Streptococcus dysgalactiae subsp equisimilis in a hospital in the south of Brazil. Braz J Microbiol 38:417–420. doi: 10.1590/S1517-83822007000300006. [DOI] [Google Scholar]
- 20.Cherkaoui A, Emonet S, Fernandez J, Schorderet D, Schrenzel J. 2011. Evaluation of matrix-assisted laser desorption ionization-time of flight mass spectrometry for rapid identification of beta-hemolytic streptococci. J Clin Microbiol 49:3004–3005. doi: 10.1128/JCM.00240-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beall B, Facklam R, Thompson T. 1996. Sequencing emm-specific PCR products for routine and accurate typing of group A streptococci. J Clin Microbiol 34:953–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicholson ML, Ferdinand L, Sampson JS, Benin A, Balter S, Pinto SWL, Dowell SF, Facklam RR, Carlone GM, Beall B. 2000. Analysis of immunoreactivity to a Streptococcus equi subsp. zooepidemicus M-like protein to confirm an outbreak of poststreptococcal glomerulonephritis, and sequences of M-like proteins from isolates obtained from different host species. J Clin Microbiol 38:4126–4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawakami V, Rietberg K, Lipton B, Eckmann K, Watkins M, Oltean H, Meagan Kay M, Rothschild C, Kobayashi M, Beneden CV, Duchin J. 2016. Notes from the field. Fatal infection associated with equine exposure–King County, Washington, 2016. MMWR Morb Mortal Wkly Rep 65:788. [DOI] [PubMed] [Google Scholar]
- 24.Le-Berre N, Filipozzi P, Martin L, Frimat L, Girerd S. 2017. First case report of Streptococcus equi subsp. zooepidemicus post-infectious acute glomerulonephritis in France. Nephrol Ther 13:37–41. doi: 10.1016/j.nephro.2016.07.448. [DOI] [PubMed] [Google Scholar]
- 25.Veldeman L, De Wilde K, Vogelaers D, Lerut E, Vonck A, Mertens D, Koch A, Beckers J. 2018. Acute renal failure with need for renal replacement therapy as a complication of zoonotic S. zooepidemicus infection: case report and review of the literature. Acta Clin Belg 73:144–150. doi: 10.1080/17843286.2017.1326547. [DOI] [PubMed] [Google Scholar]
- 26.Kittang BR, Pettersen VK, Oppegaard O, Skutlaberg DH, Dale H, Wiker HG, Skrede S. 2017. Zoonotic necrotizing myositis caused by Streptococcus equi subsp. zooepidemicus in a farmer. BMC Infect Dis 15:147. doi: 10.1186/s12879-017-2262-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duca E, Teodorovici GR, Radu C, Vita A, Talasman-Niculescu P, Bernescu E, Feldi CC, Rosca V. 1969. A new nephritogenic Streptococcus. J Hyg(Lond)67:691–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mcmillan DJ; Dréze P, Vu T, Bessen DE, Guglielmini Steer J. 2013. Updated model of group A Streptococcus M proteins based on a comprehensive worldwide study. Clin Microbiol Infect 19:E222–E229. doi: 10.1111/1469-0691.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anzai T, Walker JA, Blair MB, Chambers TM, Timoney JF. 2000. Comparison of the phenotypes of Streptococcus zooepidemicus isolated from tonsils of healthy horses, specimens obtained from foals, donkeys with pneumonia. Am J Vet Res 61:162–166. doi: 10.2460/ajvr.2000.61.162. [DOI] [PubMed] [Google Scholar]
- 30.Beres SB, Sesso R, Pinto SWL, Hoe NP, Porcella SF, Deleo FR, Musser JM. 2008. Genome sequence of a Lancefield group C Streptococcus zooepidemicus strain causing epidemic nephritis: new information about an old disease. PLoS One 21:e3026. doi: 10.1371/journal.pone.0003026. [DOI] [PMC free article] [PubMed] [Google Scholar]