Abstract
Purpose: Obesity as a serious public health problem worldwide, results in the incidence of many chronic diseases. Obesity has been recognized as a chronic low-grade inflammation disorder. Altered endocannabinoid system tone is also involved in the pathogenesis of obesity. The present study aimed to investigate the effects of oleoylethanolamide supplementation on inflammatory biomarkers and oxidative stress in obese people.
Methods: This randomized, double-blind, placebo-controlled clinical trial was carried out on 60 healthy obese people in 2016 in Tabriz, Iran. Eligible subjects were randomly divided into intervention (received daily, two 125 mg OEA capsules) and control groups (the same amounts of starch) and treated for 8 weeks. Blood samples (5 ml) were taken in fasting state at the baseline and at the end of the study. The concentrations of MDA and TAS were measured using a spectrophotometer. A high sensitive-C reactive protein level was measured by Immunoturbidimetry assay using the commercial kit. IL-6 and TNF-α levels were assayed by the ELISA method. The differences between groups were assessed by ANCOVA and statistical significance was determined at p<0.05.
Results: Analysis was done on 56 participants who continued intervention until the end of the study. A significant decrease in the IL-6 and TNF-α serum concentrations was observed in the intervention group (p<0.001). Changes in other variables were undetectable (p>0.05).
Conclusion: The use of OEA as a complementary pharmacotherapy agent could be effective in improving inflammation and oxidative stress in obese people. Future studies are needed to confirm the obtained results.
Keywords: Inflammation, Endocannabinoids, Obesity, Oleoylethanolamide, Oxidative stress
Introduction
Obesity as a serious public health problem, results from an imbalance between energy intake and energy expenditure.1 Growing evidences have shown that there is an increase in the prevalence of obesity in the world.2 Iran is no exception in this regard. According to the second national integrated micronutrient survey in 2011 to 2015, about 40.3 and 19.2% of the Iranian population were overweight and obese, respectively.3 Obesity was recognized as a chronic low-grade inflammation.4 Chronic inflammation participates in the appearance of many adverse disorders such as diabetes, metabolic syndrome, some kinds of cancer and nonalcoholic fatty liver disease.5-8 It has been shown that the levels of pro-inflammatory cytokines and acute phase proteins such as C-reactive protein (CRP), interleukin-6 (IL-6) and tumor necrosis factor–alpha (TNF-α) increase in obese people.9-11
Besides inflammation, an altered endocannabinoid system tone is involved in the pathogenesis of obesity.12 Endocannabinoids exert their physiological functions in the body mainly via two receptors: cannabinoid receptors 1 and 2 (CB1 amd CB2).13 Some endocannabinoids-like compounds including oleoylethanolamide (OEA) are structurally similar to endocannabinoids but are incapable of binding to cannabinoid receptors.14
Oleoylethanolamide as an endogenous ethanolamide fatty acid is synthesized in the small intestine cells and adipose tissues,15,16 neurons17 and astrocytes18 and exerts its biological functions through other pathways instead of cannabinoid receptors.19 Oatmeal, nuts, and cocoa powder are the major foods sources of OEA in meals. However, the amount of OEA found in these foods is low (fewer than 2 µg/g).20,21 In addition to the protective effects of OEA in the many metabolic diseases including neurodegenerative disorders, anti-atherosclerosis effects, apoptosis, relieving pain, enhancement of lipid metabolism, cytoprotective actions on the pancreatic beta cells,22-34 decreasing inflammation and body weight are considered as other beneficial effects of OEA.25-35 In obese people, OEA can regulate the energy homeostasis and appetite mainly by activation of various receptors including proximal proliferator-activated receptor-α (PPAR-α), G-protein-coupled receptor 119 (GPR119) and transient receptor potential cation channel subfamily V (TRPV1). Indeed, OEA activates these receptors and delays meal initiation, reduces meal size, decreases intervals between meals and finally modulates body weight.23,36-38
According to experimental studies, OEA also suppresses the expression of IL-6, interleukin-8 (IL-8), intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) in TNF-α induced inflammation in human umbilical vein endothelial cells through the activation of inflammatory receptors. OEA also inhibited the nuclear factor kappa-B (NF-kB) pathway in the body.39 In YT et al's survey, OEA (50 µmol/L) inhibited the TNF-α induced VCAM-1 expression in HUVEC.40
Considering the high prevalence of obesity and due to the lack of clinical studies assessing OEA´s potential in reducing inflammatory biomarkers and oxidative stress in obese people, the present study was aimed at investigating the effects of OEA supplementation on the inflammation and oxidative stress biomarkers in obese people.
Materials and Methods
This randomized, double-blind, controlled clinical trial was done on 60 healthy obese people from November to May 2016 in Tabriz, Iran. Individuals between the ages of 18 and 59 years and with a body mass index (BMI) between 30 and 40 kg/m2 were defined as inclusion criteria. All participants were informed through the announcement and poster invitation and were recruited from clinics and healthcare centers of Tabriz University of Medical Science. Exclusion criteria were participants with current clinical problems including kidney diseases, liver and heart failure, gastrointestinal and rheumatic disorders, cigarette, smoking, pregnancy, and breastfeeding and menopause in women, participants taking antibiotics, probiotics and prebiotics supplements, weight loss drugs, omega 3 supplements, and multivitamin and mineral supplements during one month prior to the study.
The sample size was calculated according to the same variable (body mass) as a previous study.41 By considering the confidence interval 95%, power 90%, two-tailed test, and taking into account the 0.9% changes, the minimum sample size was calculated to be 26 healthy obese people in each group. Given that losses were possible in the follow-up period, 30 individuals were included in each group.
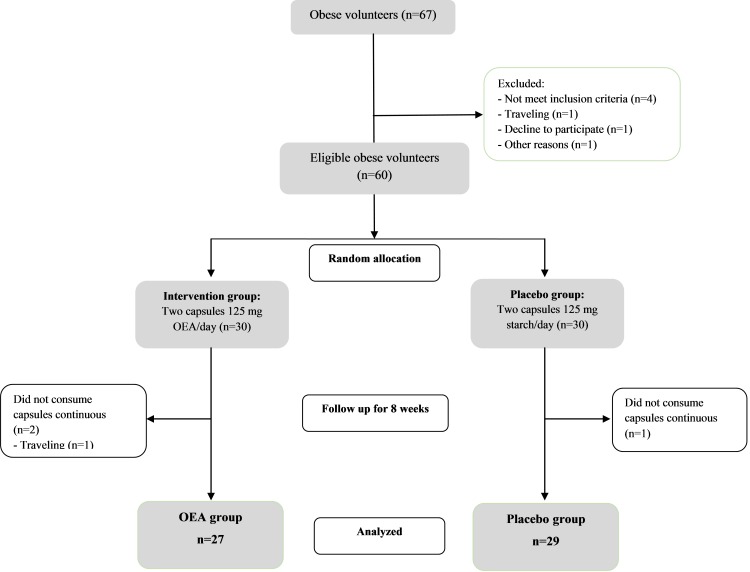
A written consent form completed by eligible participants at baseline. Demographic questionnaires including age, sex, occupational status, and educational level were completed by participants at baseline.All participants were randomly allocated to an intervention or a placebo group. The intervention group received two 125 mg OEA capsules daily 30-minutes before lunch and dinner meals (synthesized at the Nutrition Research Center, Tabriz University of Medical Science, Iran); the materials for synthesis were obtained from the Sigma-Aldrich Company (in detail was explained in our previous study)42 for 8 weeks. The placebo group received the same amount of placebo (starch). A third person who labeled capsules with identical shapes and colors with 2 codes remained unknown to the researchers until the end of the assays. Figure 1 shows how people were recruited and followed until the end of the study. All subjects were followed by weekly phone call to confirm that they consumed the capsules regularly. The data on those who consumed more than 90% of the capsules were analyzed.
Figure 1.
The flowchart of the study from baseline until the end of study
Anthropometric and biochemical assessments
At baseline, anthropometric measurements were taken in the fasting state. Body weights and heights were measured without shoes and with light clothing using a Seca scale (precision 100 g, Seca, Hamburg, Germany) and a stadiometer (Seca, Germany), respectively. The BMI was calculated by dividing weight/height2 (kg/m2).
Blood samples (5mL) were collected in vacuum collection tubes containing EDTA in the fasting state (10-12 hour, water permitted) at baseline and at the end of the study. The serums were isolated by high-speed centrifugation and were frozen immediately at −70 °C until assay. The concentration of malondialdehyde (MDA) and the total antioxidant status (TAS) were measured with a spectrophotometer using a commercial kit (Merck chemicals and Randox Laboratories, Ltd, Crumlin, UK, respectively). The Immunoturbidimetry assay and commercial kit were used to measure the high sensitive-C reactive protein level (Biosystems, Barcelona Spain COD 31927). The levels of IL-6 and TNF-α were also assayed using ELISA kits, according to the manufacturer’s instructions (IBL (REF BE53061 and REF BE55001, International GmbH, Hamburg, Germany).
Statistical analysis
SPSS software was used to analysis of data (version 20; SPSS Inc., Chicago, IL). The normal distribution of variables was assessed by the Kolmogorov-Smirnov test. Numerical data was presented as mean (Standard deviation) and categorical data was presented as frequency (percentage). The baseline characteristics were compared by independent sample t tests (for quantitative variables) and the chi-squared test (for qualitative variables) in both groups. The within-group changes of IL-6, TNF-α, hs-CRP, MDA, and TA concentrations were assessed by paired sample t-test. We used analysis of covariance (ANCOVA) to detect difference between the intervention and placebo groups after adjusting for baseline measurements and confounder factors including age, sex, occupational and educational status. p< 0.05 was defined as statistically significant.
Results and Discussion
Table 1 presents the baseline characteristics of participants in both groups. The analysis was performed on data from 56 participants who completed the intervention. Mean (SD) ages of subjects in the intervention group were 37.37 (8.74) and in the placebo group was 38.13 (9.28) years. About 55% and 65% of subjects in the intervention and placebo groups were females. No significant differences detected for all baseline variables with the exception of educational level (p>0.05). Participants reported no side effect or symptoms either during OEA treatment or at the end of the intervention.
Table 1. The demographic characteristics of obese people in the onset of study (n=56) .
| Variables | OEA group (n=27) | Placebo group (n=29) | P a |
| Age (year) | 37.37 (8.74) | 38.13 (9.28) | 0.572 |
| Gender | |||
| - Female | 15 (55.6) | 19 (65.5) | 0.109 |
| - Male | 12 (44.4) | 10 (34.5) | |
| Education level | |||
| - Illiterate | 13 (48.1) | 15 (51.7) | 0.010 |
| -Diploma | 3 (11.1) | 11 (37.9) | |
| - Bachelor degree and above | 11 (40.7) | 3(10.3) | |
| Occupation | |||
| - Clerk | 8 (29.6) | 4 (13.8) | 0.438 |
| - Housewife | 14 (51.9) | 19 (65.5) | |
| - Worker | 5 (18.5) | 6 (20.7) | |
| Weight (kg) | 93.02 (13.22) | 91.23 (13.61) | 0.628 |
| Height (cm) | 163.73 (9.41) | 160.96 (8.91) | 0.340 |
| BMI (kg/m2) | 34.69 (2.41) | 35.11 (2.82) | 0.552 |
| IL-6 (pm/mL) | 6.51 (0.95) | 6.43 (0.64) | 0.698 |
| TNF-α (pm/mL) | 6.44 (3.19) | 5.86 (1.85) | 0.412 |
| Hs-CRP (pm/mL) | 7.04 (3.12) | 7.28 (2.23) | 0.666 |
| TAS (mmol/L) | 1.56 (0.37) | 1.52 (0.37) | 0.682 |
| MDA (µmol/L) | 1.79 (0.49) | 1.72 (0.30) | 0.533 |
a Independent sample t-Test/ Chi2 test
Data presented as Mean (SD)/ frequency (%)
Figure 2 shows the changes in the inflammatory biomarkers and oxidative stress indices before and after the intervention in both groups. The levels of IL-6 decreased significantly in the intervention group (before Mean (SD) = 6.51 (0.95), after Mean (SD) = 5.99 (0.72); p=0.011). Changes in the placebo group were not significant (p>0.05). According to the ANCOVA test, there was a significant difference between groups regarding the IL-6 variable, after adjusting for baseline value and demographic characteristics (p<0.001). TNF-α decreased significantly in the OEA group in comparison with baseline (before Mean (SD)=6.44 (3.19), after Mean (SD)=4.20 (2.19); p=0.007) and this result was confirmed by ANCOVA test (p=0.001).
Figure 2.
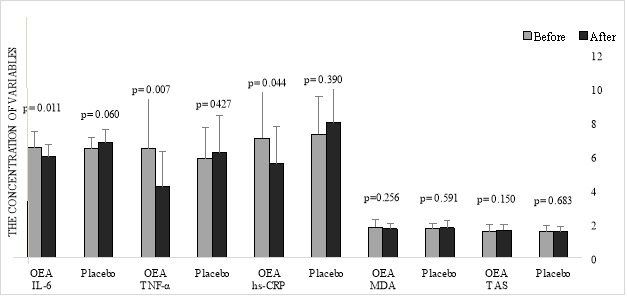
The Mean (SD) of inflammatory biomarkers and oxidative stress indices in the OEA and placebo groups (n=56)
The levels of hs-CRP in the OEA group decreased significantly at the end of the study (p=0.044) and a non-significant increase of this variable was detected in the placebo group (p>0.05). However, the ANCOVA test did not attain statistical significance. Changes in other biomarkers (MDA and TAS) were not significant (p>0.05). Table 2 presents the mean difference between inflammatory biomarkers and oxidative stress indices between groups after adjusting for baseline value and demographic characteristics.
Table 2. The effects of OEA supplementation on the inflammatory biomarkers and oxidative stress in obese people (n=56) .
| Variables | OEA group (n=27) | Placebo group (n=29) |
Mean Diff (95% CI)
P a |
| IL-6 (pm/mL) | |||
| Before | 6.51 (0.95) | 6.43 (0.64) | 0.853 (0.447-1.258) <0.001 |
| After | 5.99 (0.72) | 6.77 (0.78) | |
| TNF-α (pm/mL) | |||
| Before | 6.44 (3.19) | 5.86 (1.85) | 2.127 (0.935-3.319) 0.001 |
| After | 4.20 (2.09) | 6.21 (2.20) | |
| hs-CRP (pm/mL) | |||
| Before | 7.04 (3.12) | 7.28 (2.23) | 1.590 (-0.242-3.422) 0.087 |
| After | 5.56 (2.16) | 7.97 (2.41) | |
| MDA (µmol/L) | |||
| Before | 1.79 (0.49) | 1.72 (0.30) | 0.108 (-0.085-0.300) 0.267 |
| After | 1.70 (0.33) | 1.76 (0.41) | |
| TAS (mmol/L) | |||
| Before | 1.56 (0.37) | 1.52 (0.37) | 0.093 (-0.210-0.025) 0.119 |
| After | 1.63 (0.32) | 1.53 (0.34) |
Data were presented as Mean (SD)
a Mean Diff (95% CI)P for ANCOVA test (Adjusting on Baseline values, age, gender, education, and occupation variables)
Different dietary interventions have been identified to reduce inflammatory cytokines in chronic disorders.43 Indeed, dietary components act as ligands of the receptors for the activation of genes involved in the regulation of inflammatory responses.44 Oleoylethanolamide, a lipid mediator component, is a structural analog of the endocannabinoids with several beneficial effects in metabolic status.41,45
The present study investigated the potential anti-inflammatory effects of OEA in obesity. At the end of the study, the concentrations of IL-6 and TNF-α decreased significantly. However, the level of other biomarkers did not show significant changes between groups. Higher levels of IL-6 in obese people increases the expression of other acute phase proteins and systemic inflammation markers such as CRP.46 In agreement with this study, Anton et al investigated the anti-inflammatory effects of OEA in 87 rats exposed to alcohol. Supplementation of male Wistar rats (n=4-6 per group) with 5mg/kg OEA, resulted in a decrease of interleukin-1 β (IL-1 β), monocyte chemoattractant protein 1 (MCP-1), TNF-α levels and inhibition of pro-inflammatory enzymes including cyclooxygenase-2 (COX-2) and inducible nitric oxide syntheses (iNOS). Also, it has been reported that OEA had anti-depression effects and neuroprotective effects on alcohol intoxicated rats.35 In the study of Sun et al, the neuroprotective effects of OEA through the activation of PPAR-α receptors were reported. Supplementation of OEA (10mg/kg) increased the expression of IkB as NFkB-inhibitory protein in cortex cerebral mouse and inhibited the expression of COX-2 as NFkB-regulated protein.26 Zolese et al investigated the effects of OEA on lipid peroxidation and high-density lipoprotein (HDL)-associated paraoxonase activity (as an enzyme involved in antioxidant and anti-inflammatory pathways) in plasma samples derived from 13 healthy people. The results showed that incubation of plasma with 1µM OEA and 20-70µM CuSO4 and 10 mM (2,2 -Azobis-(2-amidinopropane) hydrochloride (AAPH) for 7 h protected plasma lipids and paraoxonase 1 enzyme activity against AAPH and copper-induced oxidation.47
In another study, the anti-inflammatory and neuroprotective properties of OEA were assessed in neuro-inflamed Wistar Hannover rats induced by lipopolysaccharide (LPS) (n=94, 5-6/group). OEA (10 mg/kg) prevented the significant up-regulation of NFkB and significantly reduced lipopolysaccharide-induced oxidative/nitrosative stress (p<0.001). Also, OEA disrupted lipopolysaccharide-induced anhedonia as the main symptom of a depressive-like state (p<0.001).48 In the study of Ma et al, the antioxidant and anti-inflammatory properties of OEA in 20 male mice were investigated. OEA (10 mg/kg/day) was administered orally for 8 weeks. The results showed that OEA prevented oxidative stress and endothelial cell damage, and inhibited early atherosclerotic plaque formation.49Table 3 summarized the results of studies regarding OEA supplementation on the inflammatory biomarkers and oxidative stress.
Table 3. The results of studies about the effects of oleoylethanolamide on the inflammatory biomarkers and oxidative stress .
| Samples | Dose, Duration | Results | Reference |
| Wild-type mice (C57BL/6) | OEA (10mg/kg) for 3 days | - The expression of IkB as NFkB-inhibitory protein in cortex cerebral of mouse increased - The expression of COX-2 as NFkB-regulated protein inhibited |
Sun et al. (2007) |
| Healthy people (n=13) | OEA (1µM) CuSO4 (20-70µM) (2,2 -Azobis-(2-amidinopropane) hydrochloride (AAPH) (10 mM) for 7 hours |
- Plasma lipids and paraoxonase 1 enzyme activity protected against AAPH and copper-induced oxidation by OEA | Zolese et al. (2008) |
| Vascular endothelial cell, healthy HUVEC and TNF-alpha induced HUVECs | OEA (10, 50 and 100 µmol.L) cultured at 37 degrees C for 7 h | - OEA (10 and 50 µmol.L) induced the CB2 protein and mRNA expression, but not 100 µmol.L. - TNF-alpha induced VCAM-1 expression and THP-1 adhere to HUVEC inhibited |
YT et al, (2014) |
| Male mice (n=20) | OEA (10 mg/kg) administered orally 8 weeks |
- Oxidative stress and endothelial cell damage prevented - Early atherosclerotic plaque formation inhibited |
Ma et al. (2015) |
| Wistar Hannover rats (n=94, 5-6 /group) terated by LPS |
OEA (10 mg/kg) 8 weeks |
- Up-regulation of NFkB/ lipopolysaccharide-induced oxidative/nitrosative stress inhibited significantly (p<0.001) - Lipopolysaccharide-induced anhedonia as a core symptom of a depressive-like state disrupted (p<0.001) |
Sayd et al, (2015) |
| Wistar rats exposed to alcohol (n=87, n=4-6 per groups) | OEA (5mg/kg) |
- The levels of IL-1β, MCP-1, TNF-α decreased - Pro-inflammatory enzymes including COX-2 and iNOS inhibited - OEA had anti-depression effects and neuroprotective effects on alcoholic rats |
Anton et al. (2017) |
OEA exert its antioxidant and anti-inflammatory effects through different mechanisms. OEA acts as an endogenous ligand of the PPAR-α.42,50 PPAR-α is a member of the nuclear receptor proteins family and exerts effective roles in many metabolic pathways such as adipocytes differentiation and lipid metabolism.51 Regulating inflammatory responses was also attributed to the PPAR-α function. In macrophages, PPAR-α inhibits the scavenger receptor and nitric oxide synthesis.39 Also, PPAR-α reduces leukocyte adhesion to the activated endothelial cells and controls the migration of transendothelial leukocytes.52 Agonists of PPAR-α suppress the expression of IL-6 and COX-2 involved in the inflammatory pathways.53 OEA as a ligand of PPAR-α binds to the PPAR-α receptors and reduces the production of reactive oxygen species (ROS) and pro-inflammatory cytokines.54 OEA also inhibits the expression of VCAM-1 and ICAM-1 as molecules involved in the inflammatory responses.55 Other mechanisms involved in the cytoprotective and anti-inflammatory effects of OEA include the increasing activity of the anti-oxidative enzyme, reducing lipid peroxidation, and apoptosis-related proteins expression.49
To the best of the authors´ knowledge, this was the first clinical study to evaluate the unique effects of oleoylethanolamide on the inflammatory biomarkers and oxidative stress in obese people. The main limitations of this study include the insufficient purity of the synthesized OEA (~ 85%), not measuring OEA concentrations in the serum samples and the shorter duration of the supplementation.
Conclusion
In summary, daily supplementation of two 125 mg oleoylethanolamide in 56 healthy obese people for 8 weeks significantly decreased the levels of IL-6 and TNF-α; however, changes in MDA, TAS, and hs-CRP were insignificant. Considering the many beneficial effects of OEA, its use as a complementary agent could be effective in improving inflammation in obese people. However, future studies are needed to confirm the current results.
Acknowledgments
The authors thank the Department of Nutrition, Faculty of Health and Nutrition. This is a part of a database from Ph.D. thesis entitled "The effect of oleoylethanolamide supplementation on PPAR-α gene expression, some inflammatory biomarkers and the abundance of Akkermansia muciniphila bacteria in the stool of obese people: A double-blind randomized placebo-controlled clinical trial".
Ethical Issues
A written consent form completed by eligible subjects. The regional ethics committee of the Tabriz University of Medical Science approved the whole protocol of the study and allocated the number code IR.TB MED.REC.1395.618. The study was registered in the Iranian Registry of Clinical trial center with number IRCT201607132017N30 with URL: www.IRCT.IR.
Conflict of Interest
The authors declare there is no conflict of interest in the content of this study.
References
- 1.Fallah-Fini S, Rahmandad H, Huang TT, Bures RM, Glass TA. Modeling us adult obesity trends: A system dynamics model for estimating energy imbalance gap. Am J Public Health. 2014;104(7):1230–9. doi: 10.2105/AJPH.2014.301882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aekplakorn W, Inthawong R, Kessomboon P, Sangthong R, Chariyalertsak S, Putwatana P. et al. Prevalence and trends of obesity and association with socioeconomic status in thai adults: National health examination surveys, 1991-2009. J Obes. 2014;2014:410259. doi: 10.1155/2014/410259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pouraram H, Djazayery A, Mohammad K, Parsaeian M, Abdollahi Z, Dorosty Motlagh A. et al. Second national integrated micronutrient survey in Iran: Study design and preliminary findings. Arch Iran Med. 2018;21(4):137–44. [PubMed] [Google Scholar]
- 4.Calder PC, Ahluwalia N, Brouns F, Buetler T, Clement K, Cunningham K. et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br J Nutr. 2011;106(S3):S5–78. doi: 10.1017/S0007114511005460. [DOI] [PubMed] [Google Scholar]
- 5.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121(6):2111–7. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vucenik I, Stains JP. Obesity and cancer risk: Evidence, mechanisms, and recommendations. Ann N Y Acad Sci. 2012;1271:37–43. doi: 10.1111/j.1749-6632.2012.06750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jung UJ, Choi MS. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014;15(4):6184–223. doi: 10.3390/ijms15046184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Onuora S. Rheumatoid arthritis: Obesity skews markers of inflammation. Nat Rev Rheumatol. 2017;13(6):323. doi: 10.1038/nrrheum.2017.62. [DOI] [PubMed] [Google Scholar]
- 9.Lukic L, Lalic NM, Rajkovic N, Jotic A, Lalic K, Milicic T. et al. Hypertension in obese type 2 diabetes patients is associated with increases in insulin resistance and il-6 cytokine levels: Potential targets for an efficient preventive intervention. Int J Environ Res Public Health. 2014;11(4):3586–98. doi: 10.3390/ijerph110403586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Derosa G, Fogari E, D’Angelo A, Bianchi L, Bonaventura A, Romano D. et al. Adipocytokine levels in obese and non-obese subjects: An observational study. Inflammation. 2013;36(4):914–20. doi: 10.1007/s10753-013-9620-4. [DOI] [PubMed] [Google Scholar]
- 11.Maachi M, Pieroni L, Bruckert E, Jardel C, Fellahi S, Hainque B. et al. Systemic low-grade inflammation is related to both circulating and adipose tissue TNFalpha, leptin and IL-6 levels in obese women. Int J Obes Relat Metab Disord. 2004;28(8):993–7. doi: 10.1038/sj.ijo.0802718. [DOI] [PubMed] [Google Scholar]
- 12.Geurts L, Lazarevic V, Derrien M, Everard A, Van Roye M, Knauf C. et al. Altered gut microbiota and endocannabinoid system tone in obese and diabetic leptin-resistant mice: Impact on apelin regulation in adipose tissue. Front Microbiol. 2011;2:149. doi: 10.3389/fmicb.2011.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roche R, Hoareau L, Bes-Houtmann S, Gonthier MP, Laborde C, Baron JF. et al. Presence of the cannabinoid receptors, CB1 and CB2, in human omental and subcutaneous adipocytes. Histochem Cell Biol. 2006;126(2):177–87. doi: 10.1007/s00418-005-0127-4. [DOI] [PubMed] [Google Scholar]
- 14.Bellocchio L, Cervino C, Pasquali R, Pagotto U. The endocannabinoid system and energy metabolism. J Neuroendocrinol. 2008;20(6):850–7. doi: 10.1111/j.1365-2826.2008.01728.x. [DOI] [PubMed] [Google Scholar]
- 15.Schmid PC, Wold LE, Krebsbach RJ, Berdyshev EV, Schmid HH. Anandamide and other N-acylethanolamines in human tumors. Lipids. 2002;37(9):907–12. doi: 10.1007/s11745-002-0978-z. [DOI] [PubMed] [Google Scholar]
- 16.Hansen HS, Moesgaard B, Hansen HH, Petersen G. N-acylethanolamines and precursor phospholipids - relation to cell injury. Chem Phys Lipids. 2000;108(1-2):135–50. doi: 10.1016/S0009-3084(00)00192-4. [DOI] [PubMed] [Google Scholar]
- 17.Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC. et al. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372(6507):686–91. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- 18.Walter L, Franklin A, Witting A, Möller T, Stella N. Astrocytes in culture produce anandamide and other acylethanolamides. J Biol Chem. 2002;277(23):20869–76. doi: 10.1074/jbc.M110813200. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez de Fonseca F, Navarro M, Gomez R, Escuredo L, Nava F, Fu J. et al. An anorexic lipid mediator regulated by feeding. Nature. 2001;414(6860):209–12. doi: 10.1038/35102582. [DOI] [PubMed] [Google Scholar]
- 20.Astarita G, Rourke BC, Andersen JB, Fu J, Kim JH, Bennett AF. et al. Postprandial increase of oleoylethanolamide mobilization in small intestine of the burmese python (Python molurus) Am J Physiol Regul Integr Comp Physiol. 2006;290(5):R1407–12. doi: 10.1152/ajpregu.00664.2005. [DOI] [PubMed] [Google Scholar]
- 21. Premkumar LS. Fascinating facts about phytonutrients in spices and healthy food: Scientifically proven facts. Xlibris Corporation; 2014.
- 22.Gonzalez-Aparicio R, Blanco E, Serrano A, Pavon FJ, Parsons LH, Maldonado R. et al. The systemic administration of oleoylethanolamide exerts neuroprotection of the nigrostriatal system in experimental parkinsonism. Int J Neuropsychopharmacol. 2014;17(3):455–68. doi: 10.1017/S1461145713001259. [DOI] [PubMed] [Google Scholar]
- 23.Fu J, Gaetani S, Oveisi F, Lo Verme J, Serrano A, Rodriguez De Fonseca F. et al. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature. 2003;425(6953):90–3. doi: 10.1038/nature01921. [DOI] [PubMed] [Google Scholar]
- 24.Sanchis-Segura C, Spanagel R. Behavioural assessment of drug reinforcement and addictive features in rodents: An overview. Addict Biol. 2006;11(1):2–38. doi: 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- 25.Lo Verme J, Fu J, Astarita G, La Rana G, Russo R, Calignano A. et al. The nuclear receptor peroxisome proliferator-activated receptor-α mediates the anti-inflammatory actions of palmitoylethanolamide. Mol Pharmacol. 2005;67(1):15–9. doi: 10.1124/mol.104.006353. [DOI] [PubMed] [Google Scholar]
- 26.Sun Y, Alexander SP, Garle MJ, Gibson CL, Hewitt K, Murphy SP. et al. Cannabinoid activation of PPARα; a novel neuroprotective mechanism. Br J Pharmacol. 2007;152(5):734–43. doi: 10.1038/sj.bjp.0707478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mazzola C, Medalie J, Scherma M, Panlilio LV, Solinas M, Tanda G. et al. Fatty acid amide hydrolase (FAAH) inhibition enhances memory acquisition through activation of PPAR-α nuclear receptors. Learn Mem. 2009;16(5):332–7. doi: 10.1101/lm.1145209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hill MN, Miller GE, Carrier EJ, Gorzalka BB, Hillard CJ. Circulating endocannabinoids and N-acyl ethanolamines are differentially regulated in major depression and following exposure to social stress. Psychoneuroendocrinology. 2009;34(8):1257–62. doi: 10.1016/j.psyneuen.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murillo-Rodríguez E, Vázquez E, Millán-Aldaco D, Palomero-Rivero M, Drucker-Colin R. Effects of the fatty acid amide hydrolase inhibitor URB597 on the sleep-wake cycle, c-fos expression and dopamine levels of the rat. Eur J Pharmacol. 2007;562(1-2):82–91. doi: 10.1016/j.ejphar.2007.01.076. [DOI] [PubMed] [Google Scholar]
- 30.Lambert DM, Vandevoorde S, Jonsson KO, Fowler CJ. The palmitoylethanolamide family: A new class of anti-inflammatory agents? Curr Med Chem. 2002;9(6):663–74. doi: 10.2174/0929867023370707. [DOI] [PubMed] [Google Scholar]
- 31.Lueneberg K, Domínguez G, Arias-Carrión O, Palomero-Rivero M, Millán-Aldaco D, Morán J. et al. Cellular viability effects of fatty acid amide hydrolase inhibition on cerebellar neurons. Int Arch Med. 2011;4(1):28. doi: 10.1186/1755-7682-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Marzo V, Sepe N, De Petrocellis L, Berger A, Crozier G, Fride E. et al. Trick or treat from food endocannabinoids? Nature. 1998;396(6712):636–7. doi: 10.1038/25267. [DOI] [PubMed] [Google Scholar]
- 33.Stone VM, Dhayal S, Smith DM, Lenaghan C, Brocklehurst KJ, Morgan NG. The cytoprotective effects of oleoylethanolamide in insulin-secreting cells do not require activation of GPR119. Br J Pharmacol. 2012;165(8):2758–70. doi: 10.1111/j.1476-5381.2011.01755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinez de Ubago M, Garcia-Oya I, Perez-Perez A, Canfran-Duque A, Quintana-Portillo R, Rodriguez de Fonseca F. et al. Oleoylethanolamide, a natural ligand for PPAR-alpha, inhibits insulin receptor signalling in HTC rat hepatoma cells. Biochim Biophys Acta. 2009;1791(8):740–5. doi: 10.1016/j.bbalip.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 35.Antón M, Alén F, Gómez de Heras R, Serrano A, Pavón FJ, Leza JC. et al. Oleoylethanolamide prevents neuroimmune HMGB1/TLR4/NF-kB danger signaling in rat frontal cortex and depressive-like behavior induced by ethanol binge administration. Addict Biol. 2017;22(3):724–41. doi: 10.1111/adb.12365. [DOI] [PubMed] [Google Scholar]
- 36.Contreras AV, Torres N, Tovar AR. PPAR-α as a key nutritional and environmental sensor for metabolic adaptation. Adv Nutr. 2013;4(4):439–52. doi: 10.3945/an.113.003798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hansen KB, Rosenkilde MM, Knop FK, Wellner N, Diep TA, Rehfeld JF. et al. 2-Oleoyl glycerol is a GPR119 agonist and signals GLP-1 release in humans. J Clin Endocrinol Metab. 2011;96(9):E1409–17. doi: 10.1210/jc.2011-0647. [DOI] [PubMed] [Google Scholar]
- 38.Zhang LL, Yan Liu D, Ma LQ, Luo ZD, Cao TB, Zhong J. et al. Activation of transient receptor potential vanilloid type-1 channel prevents adipogenesis and obesity. Circ Res. 2007;100(7):1063–70. doi: 10.1161/01.RES.0000262653.84850.8b. [DOI] [PubMed] [Google Scholar]
- 39.Xu X, Guo H, Jing Z, Yang L, Chen C, Peng L. et al. N-Oleoylethanolamine Reduces Inflammatory Cytokines and Adhesion Molecules in TNF-alpha-induced Human Umbilical Vein Endothelial Cells by Activating CB2 and PPAR-alpha. J Cardiovasc Pharmacol. 2016;68(4):280–91. doi: 10.1097/FJC.0000000000000413. [DOI] [PubMed] [Google Scholar]
- 40.Gai YT, Shu Q, Chen CX, Lai YL, Li WJ, Peng L. et al. Anti-atherosclerosis role of N-oleoylethanolamine in CB2. Yao Xue Xue Bao. 2014;49(3):316–21. [PubMed] [Google Scholar]
- 41.Mangine GT, Gonzalez AM, Wells AJ, McCormack WP, Fragala MS, Stout JR. et al. The effect of a dietary supplement (n-oleyl-phosphatidyl-ethanolamine and epigallocatechin gallate) on dietary compliance and body fat loss in adults who are overweight: A double-blind, randomized control trial. Lipids Health Dis. 2012;11:127. doi: 10.1186/1476-511X-11-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Payahoo L, Khajebishak Y, Barzegari A, Alipour SH, Asghari Jafarabadi M, Farrin N. et al. Oleoylethanolamide increases the expression of PPAR-α and reduces appetite and body weight in obese people: A clinical trial. Appetite. 2018;128:44–9. doi: 10.1016/j.appet.2018.05.129. [DOI] [PubMed] [Google Scholar]
- 43.Payahoo L, Ostadrahimi A, Mobasseri M, Jafarabadi MA, Mahdavi AB, Mahluji S. Supplementation has anti-inflammatory effect in type 2 diabetic patients. IJTK. 2014;13(3):461–5. [Google Scholar]
- 44.Patterson E, Wall R, Fitzgerald GF, Ross RP, Stanton C. Health implications of high dietary omega-6 polyunsaturated fatty acids. J Nutr Metab. 2012;2012:539426. doi: 10.1155/2012/539426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barbaro D, Menasci A, Baldini B, Pasquini C, Lapi P. The Dietary Supplement EGCG: NOPE ( N-Oleyl-Phosphatidyletathanolamine and Epigallocatechin-3-Gallato Formula) Helps Patients to Follow a Flexible Dietary Regimen and Induces Loss of Weight in Patients who had Previously Experienced No Response to Other Weight Loss Intervention. J Obes Weig Los Ther. 2011;1(1):105. doi: 10.4172/2165-7904.1000105. [DOI] [Google Scholar]
- 46.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Low-grade systemic inflammation in overweight children. Pediatrics. 2001;107(1):E13. doi: 10.1542/peds.107.1.e13. [DOI] [PubMed] [Google Scholar]
- 47.Zolese G, Bacchetti T, Masciangelo S, Ragni L, Ambrosi S, Ambrosini A. et al. Effect of acylethanolamides on lipid peroxidation and paraoxonase activity. Biofactors. 2008;33(3):201–9. doi: 10.1002/biof.5520330306. [DOI] [PubMed] [Google Scholar]
- 48.Sayd A, Antón M, Alén F, Caso JR, Pavón J, Leza JC. et al. Systemic administration of oleoylethanolamide protects from neuroinflammation and anhedonia induced by lps in rats. Int J Neuropsychopharmacol. 2015;18(6):pii: pyu111. doi: 10.1093/ijnp/pyu111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ma L, Guo X, Chen W. Inhibitory effects of oleoylethanolamide (OEA) on H(2)O(2)-induced human umbilical vein endothelial cell (HUVEC) injury and apolipoprotein E knockout (ApoE-/-) atherosclerotic mice. Int J Clin Exp Pathol. 2015;8(6):6301–11. [PMC free article] [PubMed] [Google Scholar]
- 50.Fu J, Oveisi F, Gaetani S, Lin E, Piomelli D. Oleoylethanolamide, an endogenous PPAR-α agonist, lowers body weight and hyperlipidemia in obese rats. Neuropharmacology. 2005;48(8):1147–53. doi: 10.1016/j.neuropharm.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 51.Barger PM, Kelly DP. PPAR signaling in the control of cardiac energy metabolism. Trends Cardiovasc Med. 2000;10(6):238–45. doi: 10.1016/S1050-1738(00)00077-3. [DOI] [PubMed] [Google Scholar]
- 52.Duval C, Chinetti G, Trottein F, Fruchart JC, Staels B. The role of PPARs in atherosclerosis. Trends Mol Med. 2002;8(9):422–30. doi: 10.1016/S1471-4914(02)02385-7. [DOI] [PubMed] [Google Scholar]
- 53.Delerive P, Gervois P, Fruchart JC, Staels B. Induction of IκBα expression as a mechanism contributing to the anti-inflammatory activities of peroxisome proliferator-activated receptor-α activators. J Bioll Chem. 2000;275(47):36703–7. doi: 10.1074/jbc.M004045200. [DOI] [PubMed] [Google Scholar]
- 54.Fan A, Wu X, Wu H, Li L, Huang R, Zhu Y. et al. Atheroprotective effect of oleoylethanolamide (OEA) targeting oxidized LDL. PloS One. 2014;9(1):e85337. doi: 10.1371/journal.pone.0085337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen C, Jin X, Meng X, Zheng C, Shen Y, Wang Y. Inhibition of TNFα-induced adhesion molecule expression by (Z)-(S)-9-octadecenamide, N-(2-hydroxyethyl,1-methyl) Eur J Pharmacol. 2011;660(2-3):305–9. doi: 10.1016/j.ejphar.2011.04.009. [DOI] [PubMed] [Google Scholar]