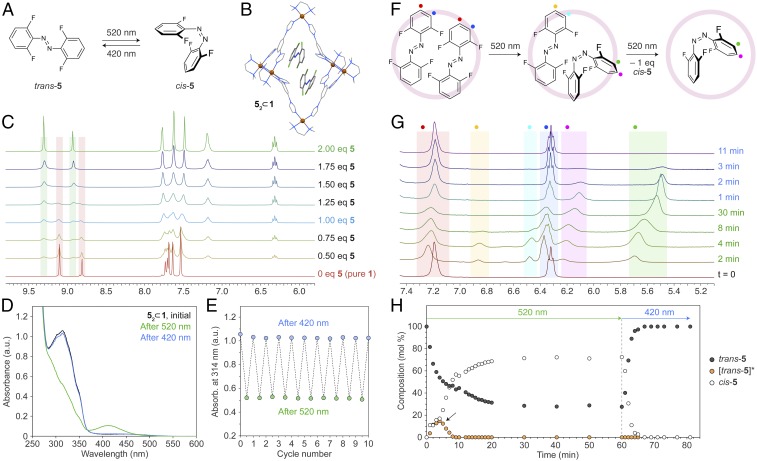
Fig. 3.
Reversible photoswitching of tetra-o-fluoroazobenzene 5 in water. (A) Green/blue light-induced isomerization of 5. (B) X-ray crystal structure of (trans-5)2 ⊂ 1 (see also SI Appendix, Fig. S60). (C) Partial 1H NMR spectra of cage 1 in the presence of increasing amounts of guest 5 (500 MHz, D2O, 298 K). The signals highlighted in red correspond to imidazole (N=CH–N) protons of empty 1 and those highlighted in green correspond to 1 filled with 2 eq of 5. (D) Changes in the UV/Vis absorption spectrum of (trans-5)2 ⊂ 1 following exposure to green light (4 min) and then to blue light (4 min). (E) Ten cycles of reversible photoisomerization of 5 within 52 ⊂ 1, followed by UV/Vis absorption spectroscopy. (F) The stepwise mechanism underlying the trans → cis photoisomerization of 52 ⊂ 1. (G) Changes in 1H NMR spectra of (trans-5)2 ⊂ 1 (bottom spectrum) during irradiation with 520-nm light (indicated in green font) for up to 30 min and subsequently with 420-nm light (blue font) for up to 11 min (500 MHz, D2O, 298 K). For a complete set of spectra, please refer to SI Appendix, Fig. S62. (H) Changes in the relative concentrations of different isomers of 5 as a function of irradiation. White markers denote encapsulated cis-5; black markers denote trans-5 within (trans-5)2 ⊂ 1; orange markers denote trans-5 within (trans-5·cis-5) ⊂ 1. The peak indicated with an arrow is due to transient intermediate (trans-5·cis-5) ⊂ 1.