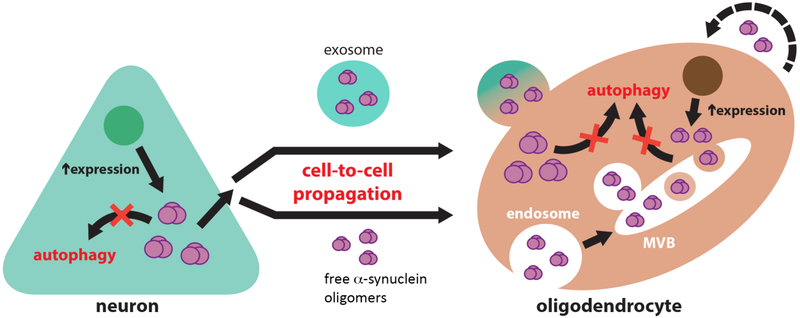
Figure 3.
Diagrammatic representation of the potential role of defective clearance in the mechanisms of α-syn transmission from neurons to oligodendroglial cells. Inhibition of autophagic clearance in neurons overexpressing α-syn may lead to an increase in its cytosolic accumulation and to the release of toxic α-syn species to the extracellular space. Extracellular α-syn may propagate from neurons to oligodendrocytes either within extracellular vesicles (exosomes) or as free-floating protein species. Oligodendrocytes are able to uptake extracellular α-syn, and due to the inhibition of oligodendroglial autophagic clearance, accumulate in the form of glial cytoplasmic inclusions at an enhanced rate. It is also possible that α-syn expression is also concurrently elevated in oligodendroglial cells, which also may release α-syn to the extracellular space in a similar fashion to neurons, thus potentiating the cell-to-cell spreading of toxic conformations of this protein.