Abstract
The aim was to describe milk feeding patterns and first weaning foods during the first year of life in a large prospective birth cohort of infants with increased genetic risk for Type 1 diabetes (T1D) recruited in 4 different countries: the United States, Finland, Germany, and Sweden. All enrolled children with dietary information (n = 8,673) were included in the analyses; 1,307 (15%) children who dropped out before the first birthday were excluded from some analyses. Supplementary milk feeding in the first 3 days of life was common in all the four countries, although the type of the supplementary milk differed by country and by maternal T1D. Donated human milk was commonly used only in Finland. In all the countries, the most common first supplementary food was cow's milk‐based infant formula, especially among offspring of mothers with T1D. The use of specific types of infant formulas differed notably by country: Extensively hydrolysed formulas were most used in Finland, partially hydrolysed ones in the United States and in Germany, and soy formulas only in the United States. Infant formulas commonly included probiotics, prebiotics, and starches. During the first year of life, most of the infants received conventional cow's milk. Overall, milk feeding during the first 3 days of life and thereafter until the first birthday differed markedly by maternal T1D status and across countries. These descriptive data may be useful in understanding early infant feeding practices and in planning potential interventions, which affect infant feeding.
Keywords: breastfeeding, infant, infant feeding, infant formula, milk feeding patterns, type 1 diabetes
Key messages.
Giving supplementary milk to the infant during the first 3 days was common in all the four countries.
In all the countries, the most common first supplementary food was cow's milk‐based infant formula, especially among offspring of mothers with T1D.
The use of specific types of infant formulas differed notably by country: extensively hydrolysed formulas were popular in Finland, partially hydrolysed ones in the United States and Germany, and soy formulas only in the United States.
Infant formulas commonly included probiotics, prebiotics, and starches.
During the first year of life, most infants received conventional cow's milk.
1. INTRODUCTION
Exclusive breastfeeding for at least the first 4 months of life is recommended by European and U.S. authorities (EFSA Panel on Dietetic Products, Nutrition and Allergies, 2009; European Society for Paediatric Gastroenterology Hepatology and Nutrition Committee on Nutrition et al., 2009; Greer et al., 2008), and for at least the first 6 months by the World Health Organization (WHO, 2015). WHO recommends the continuation of breastfeeding, along with complementary foods, until the age of 2 years. The definition of exclusive breastfeeding by WHO implies that the infant receives only human milk (including donated human milk) and no other liquids or solids except for oral rehydration solutions, drops, or syrups consisting of vitamins, mineral supplements, or medicines (WHO, 2008). Complementary foods defined by the WHO include any nutrient‐containing foods or liquids other than human milk given to infants at the time of weaning (WHO, 2001). Therefore, infant formula is considered a complementary food by WHO.
Infant feeding patterns have changed over recent decades and differ markedly between countries in spite of similar international recommendations (American Academy of Pediatrics, 2012; Nordic Council of Ministers, 2014; WHO, 2001; WHO, 2015). Infant formula is often the first food other than human milk to which an infant is exposed in high‐income and mid‐income countries (Erkkola et al., 2010; Grummer‐Strawn, Scanlon, & Fein, 2008; Hornell, Hofvander, & Kylberg, 2001; Nucci et al., 2017; Rebhan, Kohlhuber, Schwegler, Koletzko, & Fromme, 2009; Schiess et al., 2010). Feeding practices, for example, giving supplementary milk to the infant, on the maternity ward may affect later infant feeding patterns (Blomquist, Jonsbo, Serenius, & Persson, 1994; Erkkola et al., 2010; Kramer et al., 2001). Variation in feeding practices in maternity wards may account for differences in infant feeding habits between nations. In many countries, it has been very common to supplement human milk with infant formula while infants were still in the hospital (Blomquist et al., 1994; Erkkola et al., 2010; Grummer‐Strawn et al., 2008), although the United Nations Children's Fund (UNICEF) recommends that formula should be given to breastfed infants in the hospital only if medically indicated (UNICEF, 2015). If supplementary milk is needed on the maternity ward, donated human milk is an option where available (Schiess et al., 2010).
The feeding patterns of infants born to mothers with Type 1 diabetes (T1D) may differ for reasons related to the mother or the offspring (Hummel et al., 2007; Hummel et al., 2014). Mothers with T1D have a higher risk for giving birth by caesarean section and facing difficulties to initiate and continue breastfeeding (Hummel et al., 2007; Hummel et al., 2014; Sorkio et al., 2010). Infants of diabetic mothers are prone to hypoglycemia (Nold & Georgieff, 2004) and may therefore be more likely to receive supplementary feeding. On the other hand, both early introduction of cow's milk and a short breastfeeding duration have been reported to be associated with increased risk of clinical and preclinical T1D, but the findings remain inconsistent (H. Holmberg et al., 2007; Norris et al., 2003; Virtanen et al., 1991; Virtanen et al., 2011; Wahlberg et al., 2006). Further, the amount of cow's milk consumed in infancy and early childhood has been linked to the development of T1D in prospective studies (Lamb et al., 2015; Virtanen et al., 1998; Virtanen et al., 2000; Virtanen et al., 2012; Wahlberg et al., 2006).
The weaning process is strongly associated with family characteristics such as maternal age, education, smoking, number of siblings, and presence of diabetes in the family (Erkkola et al., 2005; Sorkio et al., 2010), as has been also observed in the current study population (Andren Aronsson et al., 2015; Hummel et al., 2014).
The aim of the current study was to describe milk feeding patterns and first weaning foods during the first year of life and to compare whether the presence of T1D in the family is related to feeding patterns in a large birth cohort of infants recruited in four different countries (the United States, Finland, Germany, and Sweden) in the Environmental Determinants of Diabetes in the Young (TEDDY) study.
2. METHODS
2.1. Study population
For the present study, we analysed infant milk feeding patterns during the first year of life of children participating in the TEDDY study. The TEDDY study is a multicentre prospective cohort study of children with increased genetic risk for T1D, where the aim is to identify environmental factors that predispose to, or protect from, beta‐cell autoimmunity and T1D. Three clinical centres in the United States and three in European countries participated. Altogether 424,788 newborn infants were genetically screened between September 2004 and February 2010, and 21,589 infants were screened positive for T1D‐associated human leukocyte antigens (HLA) genotypes, of which 8,676 enrolled in the study before the age of 4 months at the median recruitment age of 3.6 months (Hagopian et al., 2011). A detailed study design has been previously published (TEDDY Study Group, 2007; TEDDY Study Group, 2008). As of July 31, 2014, all the enrolled children in TEDDY (n = 8,676) were included in the present study, with 3 children excluded due to missing dietary information and 1,307 (15%) children excluded from certain analyses because of ended study participation before the first birthday. Of these 8,676 children, 127 (3.4%) in the United States, 68 (3.7%) in Finland, 109 (18.3%) in Germany, and 53 (2.1%) in Sweden had a mother with T1D; and 263 (7.1%) in the United States, 101 (5.5%) in Finland, 110 (18.5%) in Germany, and 118 (4.7%) in Sweden had a father or sibling with T1D. Infants of mothers with gestational diabetes were included the study's general population.
2.2. Collection of dietary data
Children identified with eligible HLA‐genotypes were invited to join the TEDDY study before 4 months of age. During the first year of the study, environmental exposures, including diet, of the participants were followed, with a clinic visit when the child was 3, 6, 9, and 12 months of age. Information about diet included the duration of exclusive and overall breastfeeding, first solid foods introduced, and the type of infant formulas used during the first year of life. The parents (or primary caretaker) recorded all the feeding information in a TEDDY notebook that was given at the 3‐month clinic visit. Clinical staff reviewed the booklet with primary caretaker at each clinic visit and entered the information into the TEDDY database. Breastfeeding duration and the infant's age at first introduction of infant formula or other complementary foods were expressed in weeks. Exclusive breastfeeding was defined to include small amounts of water and dietary supplements in addition to breast milk or donated human milk. This definition is in line with the WHO definition (WHO, 2008). The definition of overall breastfeeding included any breastfeeding, even in small amounts, and in combination with other foods.
Information about the types of infant formulas was categorized and coded into the TEDDY database by the type of protein source (cow's milk, whey only, casein only, soy, and amino acids only), by the degree of processing (nonhydrolysed, partially hydrolysed, extensively hydrolysed, or unknown) and by additions of ingredients in infant formula (e.g., starches, prebiotics, and probiotics), or by deletions of them (e.g., lactose free). Infant formulas were categorized into five groups for analysis: cow's milk‐based infant formula, extensively hydrolysed infant formula (which included amino acid‐based infant formulas), partially hydrolysed infant formula, soy formula, and unknown. The first complementary foods introduced after exclusive breastfeeding were categorized into another five groups: infant formula, root vegetables, fruit and berries, rice, and other (which included the following foods: spinach, peas/green beans, cabbages, squash/pumpkin, tomato or tomato sauce, corn, other vegetable, wheat, barley, oat, rye, pork, beef, poultry, other kinds of meat, sausage/hot dogs, fish or other seafood, egg, milk products, regular cow's milk or ice cream, and soy milk and other soy products). Feeding results regarding the first introduced complementary foods were separated into two groups, from birth and after 3 days of age, to examine which first complementary foods were introduced during the first 3 days of life (hypothetically in the maternity wards) and which came later. As the average length of time that mothers stay in the maternity ward after giving birth varies between mothers and between countries, the period of the first 3 days was chosen as a “proxy” for maternity ward stay duration so that results would be comparable between countries. The families did not receive any recommendations or advice regarding infant feeding practices during the TEDDY clinic visits.
2.3. Statistical analysis
Data was frozen as of July 31, 2014. Tables of frequencies, summary statistics, and statistical analyses were produced using Stata version 11. For analyses of exposures up to 1 year of age, only subjects who participated in TEDDY for at least 1 year were included. Data was analysed in three groups: infants of mothers with T1D, infants with a father or sibling with T1D, and the general population. As results were similar between subjects from the general population and those with a father or sibling with T1D, results are shown in the categories of infants of mothers with T1D and other infants, and by country. If these two groups did not differ, the results are shown by country. For comparisons of median duration of feeding in maternal T1D status groups by country, the Wilcoxon rank‐sum test was used, while the comparison of two population proportions was carried out using the z‐score test. Comparisons of proportions across countries for food groups, split by maternal T1D status, utilized chi‐square tests (six degrees of freedom), with Fisher's exact test when a table cell contained five or less observations.
3. RESULTS
3.1. Characteristics in relation to maternal T1D status and country
There were several differences in maternal and child characteristics by maternal T1D and by country (Table 1). All characteristics groups showed significant differences between countries, by maternal T1D group, except mother's first child, smoking during pregnancy, body mass index (only maternal T1D), and sex (both maternal T1D and father or sibling with T1D groups; p < .05). In general, mothers with T1D had a higher body mass index, more often had a caesarean section and had babies with a higher birth weight and lower Apgar scores in comparison to other mothers. There were significant differences in the median duration of exclusive and overall breastfeeding by maternal T1D and by country (Table 1).
Table 1.
Maternal and infant characteristics by maternal T1D and country in the TEDDY study (n = 8,676)
| The United States n = 3,723 | Finland n = 1,833 | Germany n = 595 | Sweden n = 2,525 | |||||
|---|---|---|---|---|---|---|---|---|
| Characteristics groupa | Mother with T1D n = 127 | Other n = 3,596 | Mother with T1D n = 68 | Other n = 1,765 | Mother with T1D n = 109 | Other n 486 | Mother with T1D n = 53 | Other n = 2,472 |
| Maternal characteristics | ||||||||
| Maternal age (years) | 29 (26–33) | 30 (26–34) | 29 (25–33.5) | 30 (27–33) | 31 (29–35) | 32 (28–35) | 31 (28–33) | 31 (28–34) |
| Maternal pre‐pregnancy BMI (kg/m2) | 24.0 (21.8–28.1) | 24.1 (21.5–28.4) | 23.9b (22.6–27.3) | 23.3 (21.1–25.9) | 23.8b (23.8–27.1) | 23.0 (20.6–26.2) | 24.8b (22.8–28.0) | 23.2 (21.2–26.3) |
| Missing data (n) | 5 | 149 | 2 | 40 | 1 | 1 | 1 | 52 |
| Mother's first child (%) | 51.5 | 40.1 | 50.9 | 44.5 | 51.8 | 48.1 | 41.5 | 47.4 |
| Missing data (n) | 26 | 946 | 13 | 344 | 26 | 172 | 12 | 548 |
| Maternal education, more than high school (%) | 92.3 | 89.7 | 98.2 | 95.3 | 92.8 | 90.1 | 75.6 | 72.4 |
| Missing data (n) | 36 | 1227 | 13 | 396 | 26 | 172 | 12 | 577 |
| Smoked during pregnancy (%) | 9.9 | 10.7 | 21.5 | 14.2 | 22.2 | 19.1 | 21.2 | 13.0 |
| Missing data (n) | 6 | 123 | 3 | 56 | 1 | 9 | 1 | 48 |
| Child characteristics | ||||||||
| Birth weight (g) | 3,582 (3,070–3,894) | 3,439 (3,070–3,750) | 3,740b (3,395–4,100) | 3,550 (3,200–3,880) | 3,615b (3,175–3,960) | 3,445 (3,100–3,740) | 3,800b (3,440–4,230) | 3,595 (3,260–3,935) |
| Missing data (n) | 8 | 226 | 0 | 4 | 0 | 0 | 0 | 1 |
| Gestational age (weeks) | 37.5b (36.0–38.0) | 40.0 (38.0–40.0) | 37b (36.2–38.2) | 40 (39.0–40.3) | 38.4b (38.0–40.0) | 40 (38.3–40.0) | 38.3b (37.7–39.6) | 40 (38.9–40.9) |
| Missing data (n) | 1 | 7 | 1 | 4 | 0 | 2 | 0 | 1 |
| 5 min Apgar score ≥ 9 (%) | 57.6b | 79.5 | 62.7b | 87.0 | 86.1b | 94.1 | 97.0 | 96.9 |
| Missing data (n) | 75 | 2461 | 1 | 21 | 1 | 13 | 20 | 893 |
| Caesarean section (%) | 67.5 b | 35.0 | 67.7 b | 15.5 | 50.5b | 31.7 | 39.6b | 14.2 |
| Missing data (n) | 75 | 2 | 0 | 4 | 0 | 0 | 0 | 0 |
| Sex, girls (%) | 46.5 | 49.5 | 63.2 | 48.6 | 53.2 | 49.6 | 47.2 | 49.5 |
| Breastfeeding duration (days) | ||||||||
| Exclusive | 0.5b (0.5–7.0) | 7.0 (0.5–56.0) | 15.0 (3.8–63.0) | 21.0 (0.5–84.0) | 7.0b (0.5–61.0) | 35.0 (0.5–137.0) | 0.5b (0.5–7.0) | 28.0 (0.5–105.0) |
| Missing data (n) | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 |
| Overall | 84.0b (28.0–280.0) | 174.0 (42.0–365.0) | 189.5 (63.0–365.0) | 247.0 (124.0–365.0) | 167.0 (35.0–314.0) | 199.5 (56.0–304.0) | 191.0 (122.0–259.0) | 210.0 (84.0–283.0) |
| Missing data (n) | 0 | 23 | 0 | 8 | 0 | 2 | 0 | 9 |
Note. Data expressed as median (interquartile range) or as %. T1D = Type 1 diabetes; TEDDY = the Environmental Determinants of Diabetes in the Young.
All characteristics groups showed significant differences (p < .05) between countries, by maternal T1D group, except mother's first child, smoking during pregnancy, BMI (only maternal T1D), and sex (both maternal T1D and father or sibling with T1D groups).
Significant difference between mothers with T1D versus other mothers within each country (p < .01).
3.2. Feeding patterns in the first 3 days of life in relation to maternal T1D status and country
There were significant differences in feeding patterns during the first 3 days of life by maternal T1D and by country (Table 2). In all countries, except Finland, infants of mothers with T1D exclusively received human milk in the first 3 days less frequently compared with others (p < .01; Table 2). Interestingly, Finland had the highest proportion of infants who exclusively received human milk but the lowest for exclusively receiving the infant's own mother's milk. Giving supplementary milk to the infant during the first 3 days was common in all four countries. Donated human milk as a supplement to the infant was common only in Finland, given at a higher frequency to infants of mothers with T1D than to infants of other mothers (91.2% vs. 66.0%, respectively, p < .01). The use of cow's milk‐based infant formula was more common among infants of mothers with T1D than among other infants (p < .01) in the first 3 days in the United States (60.6% vs. 43.4%, respectively) and Sweden (66.0% vs. 27.8%, respectively). Partially hydrolysed infant formulas were most frequently used in Germany in the first 3 days of life, and mothers with T1D used them significantly more often than other mothers (19.3% vs. 9.5%, respectively, p < .01). Examining differences between countries, feeding type was significantly associated (chi‐square p < .05) with country for the children of non‐T1D mothers. This association was also found among children of mothers with T1D, apart from feeding with extensively hydrolysed formula.
Table 2.
Proportion (%) of infants by feeding type during the first 3 days of life in mothers with T1D and other mothers by country in TEDDY study (n = 8,676)
| The United States n = 3,723 | Finland n = 1,833 | Germany n = 595 | Sweden n = 2,525 | |||||
|---|---|---|---|---|---|---|---|---|
| Feeding typea | Mother with T1D n = 127 | Other n = 3,596 | Mother with T1D n = 68 | Other n = 1,765 | Mother with T1D n = 109 | Other n = 486 | Mother with T1D n = 53 | Other n = 2,472 |
| Exclusively human milk feeding: own mother's milk and donated human milk | 33.9 | 51.3b | 75.0 | 68.4 | 51.4 | 74.5b | 28.3 | 71.3b |
| Exclusively own mother's milkc | 32.3 | 50.9b | 7.4 | 24.1 | 51.4 | 74.5b | 24.5 | 70.6b |
| Any human milk feedingd | 89.8 | 92.5 | 100.0 | 99.9 | 93.6 | 92.4 | 98.1 | 98.8 |
| Own mother's milk | 89.3 | 92.3 | 100.0 | 99.7 | 93.5 | 92.4 | 98.0 | 98.8 |
| Donated human milkd | 1.6 | 0.5 | 91.2 | 66.0b | 0.0 | 0.0 | 5.7 | 1.3b |
| Any infant formula | 61.4 | 44.9b | 22.1 | 30.1 | 44.0 | 23.9b | 69.8 | 28.2b |
| Cow's milk based formulad | 60.6 | 43.4b | 16.2 | 20.3 | 20.2 | 13.6 | 66.0 | 27.8b |
| Extensively hydrolysed formulad | 0.8 | 0.2 | 7.4 | 13.3 | 4.6 | 1.7 | 5.7 | 0.3b |
| Partially hydrolysed formulad | 1.6 | 1.8 | 0.0 | 0.0 | 19.3 | 9.5b | 0.0 | 0.2 |
| Unknown infant formulae | 2.4 | 1.3 | 0.0 | 1.3 | 0.0 | 0.0 | 1.9 | 0.1b |
Note. An infant was included in several categories, if he/she received different feeding types during the 3 first days of life. T1D = Type 1 diabetes; TEDDY = the Environmental Determinants of Diabetes in the Young.
All feeding type groups showed significant differences (p < .05) between countries, by maternal T1D group, except the extensively hydrolysed formula and unknown feeding type among infants of a mother with T1D.
Significant difference between mothers with T1D versus other mothers within each country (p < .01).
Proportion (%) of infants who were exclusively breastfed during the first 3 days (no donated human milk or other supplementary milk given).
Proportion of missing values in the human milk and donated human milk data was <1%, 1.6% in the cow's milk based infant formula data, and 2.7% in the partially hydrolysed infant formula data 27% across all countries.
Unknown group: Infant had received supplementary milk, but there were no information on what type of milk.
3.3. First introduced complementary foods and supplementary milks by country
For the next set of comparisons, we limited the TEDDY cohort to those who had been followed for at least 1 year (n = 7,366). There were differences in the first introduced complementary foods by maternal T1D status and by country (Table 3). Results are shown both from birth and after the third day of life.
Table 3.
First introduced infant formula and solid foods after exclusive breastfeeding in infants of mothers with T1D and in other infants by country in TEDDY study
| First complementary foods introduced from birth—Proportion (%) of users | ||||||||
|---|---|---|---|---|---|---|---|---|
| The United States n = 2,998 | Finland n = 1,638 | Germany n = 493 | Sweden n = 2,237 | |||||
| Food group | Mother with T1D n = 107 | Other n = 2,891 | Mother with T1D n = 62 | Other n = 1,576 | Mother with T1D n = 93 | Other n = 400 | Mother with T1D n = 48 | Other n = 2,189 |
| From birth | ||||||||
| Infant formula | 88.8 | 76.7a | 75.8 | 71.6 | 77.4 | 62.8b | 87.5 | 69.1a |
| Root vegetable | 0.0 | 2.3 | 12.9 | 12.4 | 8.6 | 18.0b | 6.3 | 15.9 |
| Fruit | 1.9 | 8.4b | 6.5 | 12.5 | 6.5 | 11.0 | 6.3 | 8.8 |
| Rice | 6.5 | 6.9 | 0.0 | 0.2 | 1.1 | 2.0 | 0.0 | 0.8 |
| Otherc | 2.8 | 5.8 | 4.8 | 3.2 | 6.5 | 6.3 | 0.0 | 5.3 |
| 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | |
| First complementary foods introduced after 3rd day of life—Proportion (%) of users | ||||||||
|---|---|---|---|---|---|---|---|---|
| The United States n = 1,613 | Finland n = 1,176 | Germany n = 358 | Sweden n = 1,633 | |||||
| Food group | Mother with T1D n = 34 | Other n = 1,579 | Mother with T1D n = 51 | Other n = 1,125 | Mother with T1D n = 51 | Other n = 307 | Mother with T1D n = 18 | Other n = 1,615 |
| After 3 days of age | ||||||||
| Infant formula | 64.7 | 58.1 | 76.5 | 60.5b | 60.8 | 51.5 | 66.7 | 58.3 |
| Root vegetable | 0.0 | 4.1 | 15.7 | 17.4 | 15.7 | 23.5 | 16.7 | 21.6 |
| Fruit | 5.9 | 15.3 | 7.8 | 17.5 | 11.8 | 14.3 | 16.7 | 12.0 |
| Rice | 20.6 | 12.5 | 0.0 | 0.3 | 2.0 | 2.6 | 0.0 | 1.1 |
| Otherc | 8.9 | 9.9 | 0.0 | 4.3 | 9.8 | 8.1 | 0.0 | 7.1 |
| 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | |
Note. Proportion (%) of users in those subjects who stayed in the study for at least the first year of life with first food information from the first year of life (n = 7,366). T1D = Type 1 diabetes; TEDDY = the Environmental Determinants of Diabetes in the Young.
Significant difference between mothers with T1D versus other mothers within each country (p < .01).
Significant difference between mothers with T1D versus other mothers within each country (p < .05).
Other group included the following foods: spinach, peas/green beans, cabbages, squash/pumpkin, tomato or tomato sauce, corn, other vegetable, wheat, barley, oats, rye, pork, beef, poultry, other meat, sausage/hot dogs, fish or other seafood, egg, milk products, regular cow's milk or ice cream, and soy milk and other soy products.
Infant formula was the most common first complementary food in all four countries. Infants of mothers with T1D more often received infant formula as the first complementary food than mothers without T1D in the United States (p < .01), Germany (p < .05), and Sweden (p < .01), but when restricting the analysis to complementary foods first consumed after the third day of life, the difference was not significant in these countries. In contrast, in Finland, there was no significant difference when looking at the data from the time of birth, but after the third day of life infants of mothers with T1D more often received infant formula as the first complementary food than infants of mothers without T1D (p < .05). Root vegetables were commonly used as the first complementary food in the European countries, but not in the United States, where rice was commonly used. For children of non‐T1D mothers, each food was significantly associated with country (p < .05) both when looking at the feeding from birth and after the third day of life. For children of mothers with T1D, the results were less consistent: Only rice and root vegetables as the first food, both from birth and from the third day of life, were significantly associated with country.
When examining the type of milk introduced first, we show results by country alone, as the type of milk did not differ between families where the mother had T1D, those with a father or sibling with T1D and those without a first degree relative with T1D. Cow's milk‐based infant formula was the most common milk type used as a first milk from birth in all the TEDDY countries, but after the third day of life, regular cow's milk (including cow's milk in baby foods/cooking and in ice cream) was the most common first milk in the United States, in Germany, and in Sweden and was also commonly used in Finland (Table 4). Similar to our observations regarding the first 3 days of life, a higher proportion of mothers in Germany introduced partially hydrolysed formulas as the first milk than mothers in the other countries, both from birth and after the third day of life. In Finland, a higher proportion of infants received extensively hydrolysed formulas as the first formula than infants in other countries did, but there was no difference between countries when excluding the first 3 days of life. Once again, virtually all milks showed a significant association with country when looking at the situation both from birth and after the third day of life, with the one exception being extensively hydrolysed milk after the third day, which did not show an association with country.
Table 4.
First introduced milks by country in TEDDY study
| Type of formula | First used supplementary milk—Proportion (%) of users | |||
|---|---|---|---|---|
| The United States n = 2,998 | Finland n = 1,638 | Germany n = 493 | Sweden n = 2,237 | |
| From birth | ||||
| Cow's milk formula | 72.9 | 62.5 | 38.5 | 71.4 |
| Extensively hydrolysed | 0.9 | 11.1 | 3.3 | 1.2 |
| Partially hydrolysed | 3.1 | 0.3 | 35.9 | 0.3 |
| Soy formula | 3.7 | 0.4 | 0.4 | 0.0 |
| Cow's milka | 13.0 | 16.5 | 16.8 | 23.8 |
| Unknownb | 1.4 | 1.3 | 0.0 | 0.1 |
| Tied/missingc | 5.0 | 8.0 | 5.1 | 3.2 |
| 100% | 100% | 100% | 100% | |
| After 3 days of age | ||||
| Cow's milk formula | 31.7 | 54.2 | 24.8 | 43.7 |
| Extensively hydrolysed | 2.5 | 2.6 | 1.0 | 2.8 |
| Partially hydrolysed | 8.1 | 0.9 | 28.8 | 0.4 |
| Soy formula | 9.9 | 0.7 | 0.4 | 0.1 |
| Cow's milka | 42.2 | 36.1 | 34.5 | 49.9 |
| Unknownb | 0.5 | 0.2 | 0.0 | 0.0 |
| Tied/missingc | 5.0 | 5.3 | 10.5 | 3.3 |
| 100% | 100% | 100% | 100% | |
Note. Proportion (%) of users in those subjects who stayed in the study for at least the first year of life with first milk information from the first year of life (n = 7,366). TEDDY = the Environmental Determinants of Diabetes in the Young.
Numbers include cow's milk, cow's milk in foods/food preparation, and ice cream.
Unknown group: Infant has received supplementary milk, but there were no information on what type of milk.
Tied/missing: Subjects were introduced to more than one supplemental milk at the same time (tied), or did not report a first supplementary milk in the first year (missing).
3.4. Types of infant formulas and milks used during the first year of life
The children received various types of infant formulas during the first year of life (Table 5). As the feeding habits were similar for subjects with a first degree relative with T1D (regardless of which relative) and those from the general population, the results are presented by country. The proportion of users of different formula types during the first year of life differed significantly between the countries. Cow's milk‐based infant formula was the most common type consumed during the first year of life in all four countries. Giving regular cow's milk or dairy products to the infant before the age of 1 year was also common, regardless of country. Similar to what was observed in the first 3 days of life and for the first introduced milks, partially hydrolysed formulas were most common in Germany, and extensively hydrolysed formulas were most common in Finland. As reported earlier (Table 4), most infants who were given an extensively hydrolysed formula received it during the first 3 days of life.
Table 5.
Proportion of users of different type of milk feeding during the first year of life by country in TEDDY study—in those subjects who stayed in the study for at least the first year of life with milk feeding information
| The United States n = 2,998 | Finland n = 1,638 | Germany n = 493 | Sweden n = 2,237 | |
|---|---|---|---|---|
| Milk feeding type | Users (%) | Users (%) | Users (%) | Users (%) |
| Cow's milk based infant formulaa | 86.1 | 88.7 | 74.8 | 78.6 |
| Soy formulab | 24.4 | 4.0 | 2.0 | 0.2 |
| Partially hydrolysed infant formulab | 19.5 | 3.7 | 49.5 | 1.0 |
| Extensively hydrolysed infant formulac | 7.3 | 22.6 | 4.1 | 6.9 |
| Cow's milkd | 98.5 | 96.7 | 95.7 | 99.2 |
| Unknowne | 2.3 | 2.0 | 0.2 | 0.1 |
Note. An infant was included in several categories, if he/she received different milk feeding types during the first year. TEDDY = the Environmental Determinants of Diabetes in the Young.
All pairs of countries differ statistically (p < .05) from each other except Germany and Sweden.
All pairs of countries differ statistically (p < .05) from each other.
All pairs of countries differ statistically (p < .05) from each other except the United States and Sweden.
All pairs of countries differ statistically (p < .05) from each other except Finland and Germany. Cow's milk variable includes cow's milk, cow's milk in foods/food preparation, and ice cream.
Unknown group: Infant has received supplementary milk, but there were no information on what kind of milk it was.
The proportion of children who received one infant formula type (the four infant formula types in the analysis were cow's milk‐based, soy, partially hydrolysed, and extensively hydrolysed infant formulas) during the first year of life was 53.6% in the United States, 65.9% in Finland, 50.9% in Germany, and 72.7% in Sweden, and the proportions receiving two infant formula types was 27.0% in the United States, 21.4% in Finland, 35.3% in Germany, and 6.8% in Sweden. The proportion of children who did not receive infant formula during the first year of life was highest in Sweden, 20.4% of children, compared with 10.0% in the United States, 9.3% in Finland, and 11.0% in Germany.
The usage of infant formulas with added probiotics during the first year of life was common in Germany but not in the other TEDDY countries (Figure 1a). Infant formulas with added prebiotics were commonly used in all the TEDDY countries (Figure 1b) and, except for in the United States, so were those with added starches (Figure 1c). The usage of infant formulas with added prebiotics became more common between 2005 and 2010 in Finland, Sweden, and the United States (Figure 1b).
Figure 1.
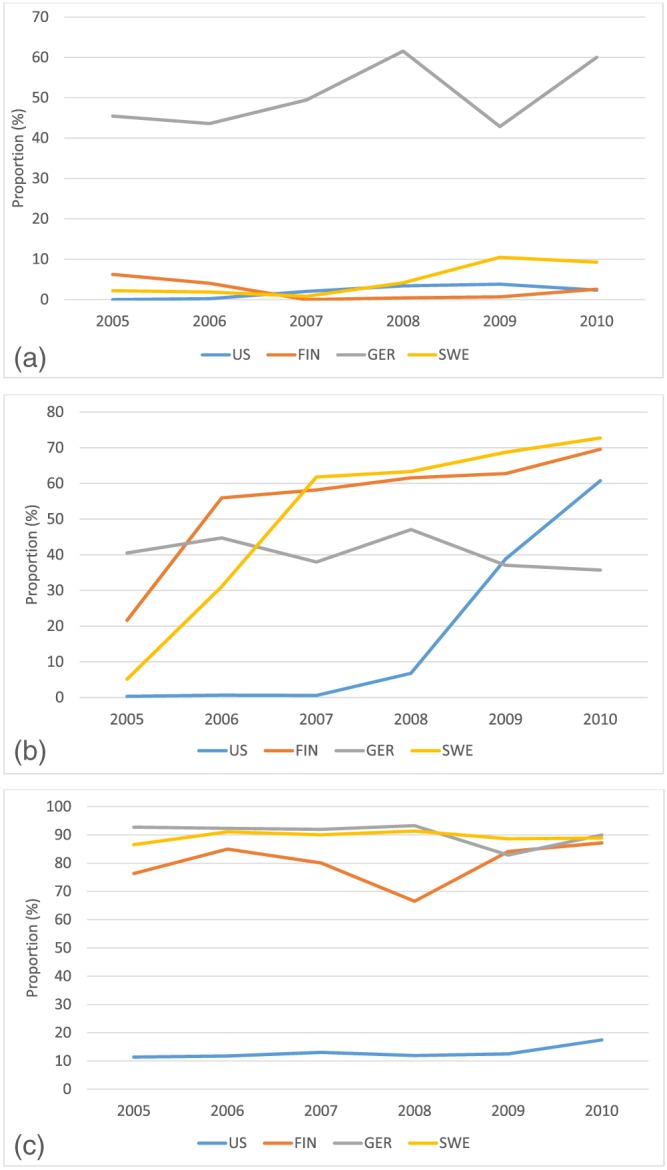
Proportion of users (%) of infant formula with added probiotics (a), prebiotics (b), and starch (c) during the first year by country in The Environmental Determinants of Diabetes in the Young (TEDDY) study. (a) Total number of infants who consumed infant formulas with added probiotics between 2005 and 2010 was 62 in the United States, 30 in Finland, 217 in Germany, and 69 in Sweden. (b) Total number of infants who consumed infant formulas with added prebiotics between 2005 and 2010 was 431 in the United States, 837 in Finland, 206 in Germany, and 1,001 in Sweden. (c) Total number of infants who consumed infant formulas with added starch between 2005 and 2010 was 333 in the United States, 1,103 in Finland, 396 in Germany, and 1,536 in Sweden
4. DISCUSSION
The results show that infant feeding patterns differ markedly by maternal T1D status and by country, from the very first days of child's life continuing through the first year. Mothers, regardless of their T1D status, in all four countries, commonly initiated breastfeeding: around 90% in the United States and Germany and nearly 100% in Finland and Sweden. At the same time, giving supplementary milk to the infant during the first 3 days, reflecting the duration of infant's stay at the maternity ward, was common in all the countries, though the type of supplementary milk differed by maternal T1D status and by country. The high frequency of giving supplementary milk to the infants during the first 3 days of life leads to the result that, of all observed infants, only 62% (ranging from 50.7% in the United States to 70.4% in Sweden) exclusively received human milk and only 52% (ranging from 23.5% in Finland to 70.3% in Germany) exclusively received their own mother's milk during that time. The most common first complementary food was infant formula in all of the countries. During the first year of life, most of the infants received conventional cow's milk (may have consumed as a drink or mixed with baby foods/cooking). Some formulas were country‐specific with soy formulas being the most popular in the United States, partially hydrolysed ones in Germany and the United States, and extensively hydrolysed ones in Finland. Infant formulas used in the current study commonly included nutritional additives: probiotics, prebiotics, and starches.
The TEDDY study is a multinational prospective birth cohort study with standardized recruitment and harmonized dietary data collection methods, which creates the opportunity to compare infant feeding habits during the first year of life according to maternal T1D status and country. The study design minimizes the possibility of recall bias due to frequent clinic visits and/or phone calls. In the data used for this report, there was a paucity of missing information (three children excluded due to missing dietary information) and a small number of children who left the study during their first year (1,307 [15%] children). The proportion of TEDDY subjects having a mother with T1D was 3.4% in the United States, 3.7% in Finland, 18.3% in Germany, and 2.1% in Sweden, which allowed us to compare infant feeding patterns between infants with and without maternal T1D.
Limitations of the study include infant feeding habits during the first 3 days being reported by parents at the first clinic visit at 3 months of age, which may have led to recall bias, and that parents do not necessarily know what kind of milk was given to their child in the maternity ward. In addition, only initiation of use, not the amount of supplementary milk was studied. All the infants participating in the study had high risk HLA‐DQ genotypes, and 11% of the infants in the study population had a mother, father, or a sibling with T1D. Germany's study population consisted of a larger proportion of infants with a first degree relative with T1D (37%) compared with the other countries. The family was informed of the infant's genetic risk 4–6 weeks after birth. The knowledge about the genetic risk, the presence of T1D in a family member, and the participation in a study protocol might have influenced feeding practices in infancy. We hypothesize that families aware of the child's increased risk of T1D may have adhered to country‐specific infant feeding guidelines more closely than the general population, especially if the family was familiar with the evidence on protective or risk factors of T1D in infancy. However, this may not be related to the first days of life (excluding T1D relatives), because the results of the genetic test were verified 4–6 weeks after birth.
In the current study, the duration of exclusive and overall breastfeeding was shorter than recommended by the WHO in all four countries. The proportion of infants who exclusively received human milk during the first 3 days was relatively low in all the countries, and even lower among infants of mothers with T1D compared with those without in the United States, Germany, and Sweden. In Finland, although the proportion of infants receiving human milk was highest, that of those receiving their own mother's milk was lowest. The reasons behind the low frequency of exclusive breastfeeding were not evaluated in the current study, and require further exploration. Lower exclusive breastfeeding rates among mothers with T1D have been observed in several studies and have been explained by demographic and clinical confounding factors such as mode of delivery, length of gestation, maternal age and education, paternal education, and early postpartum feeding (Hummel et al., 2007; Hummel et al., 2014; Sorkio et al., 2010). Sorkio et al. (2010) reported that the overall breastfeeding period was longer among infants who received only breast milk during the first 3 days, even when confounding factors were taken into account (Sorkio et al., 2010). Clinical characteristics, including caesarean section, Apgar score, and birth weight, seem to explain the lower exclusive breastfeeding rates among mothers with T1D, as previously reported in TEDDY (Hummel et al., 2014).
Our finding that a large proportion of all the infants received supplementary milk in addition to breastfeeding during the first 3 days of life in all the countries has been reported in earlier studies (Blomquist et al., 1994; Erkkola et al., 2010; Grummer‐Strawn et al., 2008; K. S. Holmberg, Peterson, & Oscarsson, 2014), although it is recommended that breastfed infants should be given supplementary milk in the maternity ward only when medically necessary (American Academy of Pediatrics, 2012; European Commission, Directorate Public Health and Risk Assessment, 2008; UNICEF, 2015). Further studies should investigate the reasons for this common use of supplementary milk during the first days of life, and what are the consequences of early supplementary feeding regarding later feeding habits. Finland differed from the other countries in the high use of donated human milk and extensively hydrolysed infant formula during the first days. The more common use of extensively hydrolysed infant formula in Finnish maternity wards might be a consequence of a dietary intervention study (TRIGR Study Group, 2007) going on in Finland, which might have had an influence on feeding practices in hospitals. The more common usage of partially hydrolysed infant formulas in Germany reflects the national recommendations for allergy prevention in infants having at least one first degree relative with an allergic disease based on the GINI‐Study (von Berg et al., 2003; von Berg et al., 2007; von Berg et al., 2008; von Berg et al., 2013).
There were differences in the first introduced milk and milk feeding during the first year of life between the participating countries. Cow's milk‐based infant formula was the most commonly used first milk type in all countries during the first year of life from birth, and also common after 3 days of age. Interestingly, after 3 days of age, the use of regular cow's milk was more frequently the first milk type than infant formula in the United States, Germany, and Sweden, and commonly used in Finland, too. This result might partly reflect the use of commercial or homemade baby foods containing, or prepared with, cow's milk.
There were country‐specific differences in the use of infant formulas with certain additions during the first year of life. While the use of formulas with added probiotics was common in Germany, but not in other countries, the use of infant formulas with added prebiotics became more prevalent in all the countries during the follow‐up of the TEDDY study. The health effects of probiotic and prebiotic products (including infant formulas) were reviewed and summarized by Thomas et al., which showed that probiotic and prebiotic products do not seem to have adverse effects for healthy infants, but more studies are needed (Thomas et al., 2010). Further, a systematic review and comment by the European Society for Pediatric Gastroenterology, Hepatology and Nutrition committee on nutrition considered that, while infant formulas are increasingly supplemented with probiotics and prebiotics, there is a need to further investigate the health effects and long‐term safety of these supplemented infant formulas (Braegger et al., 2011). According to our results, the use of infant formulas with added starches was common in European countries but not in the United States. In the United States, parents sometimes add starch (usually rice cereal) to infant formula to prevent regurgitation (O'Connor, 2009). Many commercial so‐called follow‐up formulas (from 6 months onwards) contain starch to increase the viscosity, slow down gastric emptying and increase energy density, purportedly to prolong satiety, and subsequently help babies to sleep longer. A systematic review and meta‐analysis mentioned that thickened formulas were increasingly used to treat infants with gastroesophageal reflux, an idea driven to a large extent by the baby food industry (Horvath, Dziechciarz, & Szajewska, 2008). According to that review, rice starch (more popular in North America), carob‐bean gum (more popular in Europe), carob‐seed flour, and sodium carboxymethylcellulose are often used as a thickener. Because our results indicate that the use of infant formulas with different additives is common, it calls for the investigation of their efficacy and safety. We did not assess whether parents actively decided for or against a formula with certain additives, or whether the availability in the different countries, and over time, were the main reasons for the differences between the countries in the use of infant formulas with additives.
In conclusion, milk feeding was found to differ in many respects, depending on maternal T1D and country, in the early days and during the first year of an infant's life. This descriptive data may be useful in understanding early infant feeding practices and in planning potential interventions, which affect infant feeding. This awareness may also be important for etiological research on the nutritional origin of chronic diseases.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONTRIBUTIONS
AR conducted research and wrote the manuscript; DH analysed data and contributed to the manuscript; UU, NM, SK, JY, CAA, and SH reviewed and edited the manuscript, JMN and SMV provided major input to the study design, analysis and interpretation of data, contributed to writing, and revised the article critically.
ACKNOWLEDGMENTS
The authors thank the participation of all families and the work of the TEDDY Study Group. The TEDDY Study is funded by U01 DK63829, U01 DK63861, U01 DK63821, U01 DK63865, U01 DK63863, U01 DK63836, U01 DK63790, UC4 DK63829, UC4 DK63861, UC4 DK63821, UC4 DK63865, UC4 DK63863, UC4 DK63836, UC4 DK95300, UC4 DK100238, UC4 DK106955, UC4 DK112243, UC4 DK117483, and Contract HHSN267200700014C from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute of Allergy and Infectious Diseases (NIAID), National Institute of Child Health and Human Development (NICHD), National Institute of Environmental Health Sciences (NIEHS), Centers for Disease Control and Prevention (CDC), and JDRF. This work is supported in part by the NIH/NCATS Clinical and Translational Science Awards to the University of Florida (UL1 TR000064) and the University of Colorado (UL1 TR001082).
APPENDIX 1.
THE TEDDY STUDY GROUP
Colorado Clinical Center: Marian Rewers, M.D., Ph.D., PI1,4,5,6,10,11, Kimberly Bautista12, Judith Baxter9,10,12,15, Daniel Felipe‐Morales, Kimberly Driscoll, Ph.D.9, Brigitte I. Frohnert, M.D.2,14, Marisa Gallant, M.D.13, Patricia Gesualdo2,6,12,14,15, Michelle Hoffman12,13,14, Rachel Karban12, Edwin Liu, M.D.13, Jill Norris, Ph.D.2,3,12, Adela Samper‐Imaz, Andrea Steck, M.D.3,14, Kathleen Waugh6,7,12,15, Hali Wright12. University of Colorado, Anschutz Medical Campus, Barbara Davis Center for Childhood Diabetes.
Finland Clinical Center: Jorma Toppari, M.D., Ph.D., PI¥^1,4,11,14, Olli G. Simell, M.D., Ph.D., Annika Adamsson, Ph.D.^12, Suvi Ahonen*±§, Heikki Hyöty, M.D., Ph.D.*±6, Jorma Ilonen, M.D., Ph.D.¥¶3, Sanna Jokipuu^, Leena Karlsson^, Miia Kähönenμ¤, Mikael Knip, M.D., Ph.D.*±5, Mirva Koreasalo*±§2, Kalle Kurppa, M.D., Ph.D.*±13, Tiina Latva‐ahoμ¤, Maria Lönnrot, M.D., Ph.D.*±6, Markus Mattila*, Elina Mäntymäki^, Katja Multasuoμ¤, Tiina Niininen±*12, Sari Niinistö±§2, Mia Nyblom*±, Paula Ollikainenμ¤ , Petra Rajala^, Jenna Rautanen±§, Anne Riikonen*±§, Minna Romo^, Suvi Ruohonen^, Juulia Rönkäμ¤, Satu Simell, M.D., Ph.D.¥13, Tuula Simell, Ph.D.¥12, Maija Sjöberg¥^12,14, Aino Steniusμ¤12, Sini Vainionpää^, Eeva Varjonen¥^12, Riitta Veijola, M.D., Ph.D.μ¤14, Suvi M. Virtanen, M.D., Ph.D.*±§2, Mari Vähä‐Mäkilä^, Mari Åkerlund*±§, Katri Lindfors, Ph.D.*13 ¥University of Turku, *University of Tampere, μUniversity of Oulu, ^Turku University Hospital, Hospital District of Southwest Finland, ±Tampere University Hospital, ¤Oulu University Hospital, §National Institute for Health and Welfare, Finland, ¶University of Kuopio.
Georgia/Florida Clinical Center: Jin‐Xiong She, Ph.D., PI1,3,4,11, Desmond Schatz, M.D.*4,5,7,8, Diane Hopkins12, Leigh Steed12,13,14,15, Jennifer Bryant, Janey Adams*12, Katherine Silvis2, Michael Haller, M.D.*14, Melissa Gardiner, Richard McIndoe, Ph.D., Ashok Sharma, Stephen W. Anderson, M.D.^, Laura Jacobsen, M.D.*14, John Marks, DHSc.*, P.D. Towe*. Center for Biotechnology and Genomic Medicine, Augusta University. *University of Florida, ^Pediatric Endocrine Associates, Atlanta.
Germany Clinical Center: Anette G. Ziegler, M.D., PI1,3,4,11, Andreas Beyerlein, Ph.D.2, Ezio Bonifacio Ph.D.*5, Anita Gavrisan, Cigdem Gezginci, Anja Heublein, Michael Hummel, M.D.13, Sandra Hummel, Ph.D.2, Annette Knopff7, Charlotte Koch, Sibylle Koletzko, M.D.¶13, Claudia Ramminger, Roswith Roth, Ph.D.9, Marlon Scholz, Joanna Stock9,12,14, Katharina Warncke, M.D.14, Lorena Wendel, Christiane Winkler, Ph.D.2,12,15. Forschergruppe Diabetes e.V. and Institute of Diabetes Research, Helmholtz Zentrum München, Forschergruppe Diabetes, and Klinikum rechts der Isar, Technische Universität München. *Center for Regenerative Therapies, TU Dresden, ¶Dr. von Hauner Children's Hospital, Department of Gastroenterology, Ludwig Maximillians University Munich.
Sweden Clinical Center: Åke Lernmark, Ph.D., PI1,3,4,5,6,8,10,11,15, Daniel Agardh, M.D., Ph.D.13, Carin Andrén Aronsson, Ph.D.2,12,13, Maria Ask, Jenny Bremer, Ulla‐Marie Carlsson, Corrado Cilio, Ph.D., M.D.5, Emelie Ericson‐Hallström, Annika Fors, Lina Fransson, Thomas Gard, Rasmus Bennet, Carina Hansson, Susanne Hyberg, Hanna Jisser, Fredrik Johansen, Berglind Jonsdottir, M.D., Ph.D., Silvija Jovic, Helena Elding Larsson, M.D., Ph.D. 6,14, Marielle Lindström, Markus Lundgren, M.D., Ph.D.14, Maria Månsson‐Martinez, Maria Markan, Jessica Melin12, Zeliha Mestan, Caroline Nilsson, Karin Ottosson, Kobra Rahmati, Anita Ramelius, Falastin Salami, Sara Sibthorpe, Anette Sjöberg, Birgitta Sjöberg, Carina Törn, Ph.D. 3,15, Anne Wallin, Åsa Wimar14, Sofie Åberg. Lund University.
Washington Clinical Center: William A. Hagopian, M.D., Ph.D., PI1,3,4, 5, 6,7,11,13, 14, Michael Killian6,7,12,13, Claire Cowen Crouch12,14,15, Jennifer Skidmore2, Ashley Akramoff, Jana Banjanin, Masumeh Chavoshi, Kayleen Dunson, Rachel Hervey, Rachel Lyons, Arlene Meyer, Denise Mulenga, Jared Radtke, Davey Schmitt, Julie Schwabe, Sarah Zink. Pacific Northwest Research Institute.
Pennsylvania Satellite Center: Dorothy Becker, M.D., Margaret Franciscus, MaryEllen Dalmagro‐Elias Smith2, Ashi Daftary, M.D., Mary Beth Klein, Chrystal Yates. Children's Hospital of Pittsburgh of UPMC.
Data Coordinating Center: Jeffrey P. Krischer, Ph.D.,PI1,4,5,10,11, Sarah Austin‐Gonzalez, Maryouri Avendano, Sandra Baethke, Rasheedah Brown12,15, Brant Burkhardt, Ph.D.5,6, Martha Butterworth2, Joanna Clasen, David Cuthbertson, Christopher Eberhard, Steven Fiske9, Dena Garcia, Jennifer Garmeson, Veena Gowda, Kathleen Heyman, Belinda Hsiao, Francisco Perez Laras, Hye‐Seung Lee, Ph.D.1,2,13,15, Shu Liu, Xiang Liu, Ph.D.2,3,9,14, Kristian Lynch, Ph.D. 5,6,9,15, Colleen Maguire, Jamie Malloy, Cristina McCarthy12,15, Aubrie Merrell, Steven Meulemans, Hemang Parikh, Ph.D.3, Ryan Quigley, Cassandra Remedios, Chris Shaffer, Laura Smith, Ph.D.9,12, Susan Smith12,15, Noah Sulman, Ph.D., Roy Tamura, Ph.D.1,2,13, Ulla Uusitalo, Ph.D.2,15, Kendra Vehik, Ph.D.4,5,6,14,15, Ponni Vijayakandipan, Keith Wood, Jimin Yang, Ph.D., R.D.2,15. Past staff: Michael Abbondondolo, Lori Ballard, David Hadley, Ph.D., Wendy McLeod. University of South Florida.
Project scientist: Beena Akolkar, Ph.D.1,3,4,5,6,7,10,11. National Institutes of Diabetes and Digestive and Kidney Diseases.
Other contributors: Kasia Bourcier, Ph.D.5, National Institutes of Allergy and Infectious Diseases. Thomas Briese, Ph.D.6,15, Columbia University. Suzanne Bennett Johnson, Ph.D.9,12, Florida State University. Eric Triplett, Ph.D.6, University of Florida.
Committees:
1Ancillary Studies, 2Diet, 3Genetics, 4Human Subjects/Publicity/Publications, 5Immune Markers, 6Infectious Agents, 7Laboratory Implementation, 8Maternal Studies, 9Psychosocial, 10Quality Assurance, 11Steering, 12Study Coordinators, 13Celiac Disease, 14Clinical Implementation, 15Quality Assurance Subcommittee on Data Quality.
Riikonen A, Hadley D, Uusitalo U, et al. Milk feeding and first complementary foods during the first year of life in the TEDDY study. Matern Child Nutr. 2018;14:e12611 10.1111/mcn.12611
REFERENCES
- American Academy of Pediatrics . (2012). Policy statement, breastfeeding and the use of human milk. Retrieved 02/10, 2016, from http://pediatrics.aappublications.org/content/129/3/e827
- Andren Aronsson, C. , Uusitalo, U. , Vehik, K. , Yang, J. , Silvis, K. , Hummel, S. , … The TEDDY Study Group (2015). Age at first introduction to complementary foods is associated with sociodemographic factors in children with increased genetic risk of developing type 1 diabetes. Maternal & Child Nutrition, 11(4), 803–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomquist, H. K. , Jonsbo, F. , Serenius, F. , & Persson, L. A. (1994). Supplementary feeding in the maternity ward shortens the duration of breast feeding. Acta Paediatrica (Oslo, Norway : 1992), 83(11), 1122–1126. [DOI] [PubMed] [Google Scholar]
- Braegger, C. , Chmielewska, A. , Decsi, T. , Kolacek, S. , Mihatsch, W. , Moreno, L. , … ESPGHAN Committee on Nutrition (2011). Supplementation of infant formula with probiotics and/or prebiotics: A systematic review and comment by the ESPGHAN committee on nutrition. Journal of Pediatric Gastroenterology and Nutrition, 52(2), 238–250. [DOI] [PubMed] [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (2009). Scientific opinion on the appropriate age for introduction of complementary feeding of infants. EFSA Journal, 7, 1423 Retrieved from http://www.efsa.europa.eu [Google Scholar]
- Erkkola, M. , Pigg, H. M. , Virta‐Autio, P. , Hekkala, A. , Hypponen, E. , Knip, M. , & Virtanen, S. M. (2005). Infant feeding patterns in the finnish type I diabetes prediction and prevention nutrition study cohort. European Journal of Clinical Nutrition, 59(1), 107–113. [DOI] [PubMed] [Google Scholar]
- Erkkola, M. , Salmenhaara, M. , Kronberg‐Kippila, C. , Ahonen, S. , Arkkola, T. , Uusitalo, L. , … Virtanen, S. M. (2010). Determinants of breast‐feeding in a Finnish birth cohort. Public Health Nutrition, 13(4), 504–513. [DOI] [PubMed] [Google Scholar]
- ESPGHAN Committee on Nutrition , Agostoni, C. , Braegger, C. , Decsi, T. , Kolacek, S. , Koletzko, B. , … van Goudoever, J. (2009). Breast‐feeding: A commentary by the ESPGHAN committee on nutrition. Journal of Pediatric Gastroenterology and Nutrition, 49(1), 112–125. [DOI] [PubMed] [Google Scholar]
- European Commission, Directorate Public Health and Risk Assessment . (2008). EU project on promotion of breastfeeding in Europe. Protection, promotion and support of breastfeeding in europe: A blueprint for action (revised) Retrieved 02/10, 2016, from http://www.aeped.es/sites/default/files/6-newblueprintprinter.pdf
- Greer, F. R. , Sicherer, S. H. , Burks, A. W. , American Academy of Pediatrics Committee on Nutrition , & American Academy of Pediatrics Section on Allergy and Immunology (2008). Effects of early nutritional interventions on the development of atopic disease in infants and children: The role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics, 121(1), 183–191. [DOI] [PubMed] [Google Scholar]
- Grummer‐Strawn, L. M. , Scanlon, K. S. , & Fein, S. B. (2008). Infant feeding and feeding transitions during the first year of life. Pediatrics, 122(Suppl 2), S36–S42. [DOI] [PubMed] [Google Scholar]
- Hagopian, W. A. , Erlich, H. , Lernmark, A. , Rewers, M. , Ziegler, A. G. , Simell, O. , … the TEDDY Study Group (2011). The environmental determinants of diabetes in the young (TEDDY): Genetic criteria and international diabetes risk screening of 421 000 infants. Pediatric Diabetes, 12(8), 733–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg, H. , Wahlberg, J. , Vaarala, O. , Ludvigsson, J. , & ABIS Study Group (2007). Short duration of breast‐feeding as a risk‐factor for beta‐cell autoantibodies in 5‐year‐old children from the general population. The British Journal of Nutrition, 97(1), 111–116. [DOI] [PubMed] [Google Scholar]
- Holmberg, K. S. , Peterson, U. M. , & Oscarsson, M. G. (2014). A two‐decade perspective on mothers' experiences and feelings related to breastfeeding initiation in sweden. Sexual & Reproductive Healthcare: Official Journal of the Swedish Association of Midwives, 5(3), 125–130. [DOI] [PubMed] [Google Scholar]
- Hornell, A. , Hofvander, Y. , & Kylberg, E. (2001). Introduction of solids and formula to breastfed infants: A longitudinal prospective study in Uppsala, Sweden. Acta Paediatrica (Oslo, Norway : 1992), 90(5), 477–482. [PubMed] [Google Scholar]
- Horvath, A. , Dziechciarz, P. , & Szajewska, H. (2008). The effect of thickened‐feed interventions on gastroesophageal reflux in infants: Systematic review and meta‐analysis of randomized, controlled trials. Pediatrics, 122(6), e1268–e1277. [DOI] [PubMed] [Google Scholar]
- Hummel, S. , Vehik, K. , Uusitalo, U. , McLeod, W. , Aronsson, C. A. , Frank, N. , … TEDDY Study Group (2014). Infant feeding patterns in families with a diabetes history—Observations from the environmental determinants of diabetes in the young (TEDDY) birth cohort study. Public Health Nutrition, 17(12), 2853–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel, S. , Winkler, C. , Schoen, S. , Knopff, A. , Marienfeld, S. , Bonifacio, E. , & Ziegler, A. G. (2007). Breastfeeding habits in families with type 1 diabetes. Diabetic Medicine: A Journal of the British Diabetic Association, 24(6), 671–676. [DOI] [PubMed] [Google Scholar]
- Kramer, M. S. , Chalmers, B. , Hodnett, E. D. , Sevkovskaya, Z. , Dzikovich, I. , Shapiro, S. , … PROBIT Study Group (2001). Promotion of breastfeeding intervention trial (PROBIT): A randomized trial in the Republic of Belarus. Journal of the American Medical Association, 285(4), 413–420. [DOI] [PubMed] [Google Scholar]
- Lamb, M. M. , Miller, M. , Seifert, J. A. , Frederiksen, B. , Kroehl, M. , Rewers, M. , & Norris, J. M. (2015). The effect of childhood cow's milk intake and HLA‐DR genotype on risk of islet autoimmunity and type 1 diabetes: The diabetes autoimmunity study in the young. Pediatric Diabetes, 16(1), 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nold, J. L. , & Georgieff, M. K. (2004). Infants of diabetic mothers. Pediatric Clinics of North America, 51(3), 619–637. viii [DOI] [PubMed] [Google Scholar]
- Nordic Council of Ministers (2014). Nordic nutrition recommendations 2012: Integrating nutrition and physical activity. Copenhagen: Nordic Council of Ministers. [Google Scholar]
- Norris, J. M. , Barriga, K. , Klingensmith, G. , Hoffman, M. , Eisenbarth, G. S. , Erlich, H. A. , & Rewers, M. (2003). Timing of initial cereal exposure in infancy and risk of islet autoimmunity. Journal of the American Medical Association, 290(13), 1713–1720. [DOI] [PubMed] [Google Scholar]
- Nucci, A. M. , Virtanen, S. M. , Sorkio, S. , Barlund, S. , Cuthbertson, D. , Uusitalo, U. , … TRIGR Investigators . (2017). Regional differences in milk and complementary feeding patterns in infants participating in an international nutritional type 1 diabetes prevention trial. Maternal & Child Nutrition, 13(3). 10.1111/mcn.12354. Epub 2016 Oct 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor, N. R. (2009). Infant formula. American Family Physician, 79(7), 565–570. [PubMed] [Google Scholar]
- Rebhan, B. , Kohlhuber, M. , Schwegler, U. , Koletzko, B. V. , & Fromme, H. (2009). Infant feeding practices and associated factors through the first 9 months of life in Bavaria, Germany. Journal of Pediatric Gastroenterology and Nutrition, 49(4), 467–473. [DOI] [PubMed] [Google Scholar]
- Schiess, S. , Grote, V. , Scaglioni, S. , Luque, V. , Martin, F. , Stolarczyk, A. , … European Childhood Obesity Project (2010). Introduction of complementary feeding in 5 European countries. Journal of Pediatric Gastroenterology and Nutrition, 50(1), 92–98. [DOI] [PubMed] [Google Scholar]
- Sorkio, S. , Cuthbertson, D. , Barlund, S. , Reunanen, A. , Nucci, A. M. , Berseth, C. L. , … TRIGR Study Group (2010). Breastfeeding patterns of mothers with type 1 diabetes: Results from an infant feeding trial. Diabetes/Metabolism Research and Reviews, 26(3), 206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEDDY Study Group (2007). The environmental determinants of diabetes in the young (TEDDY) study: Study design. Pediatric Diabetes, 8(5), 286–298. [DOI] [PubMed] [Google Scholar]
- TEDDY Study Group (2008). The environmental determinants of diabetes in the young (TEDDY) study. Annals of the New York Academy of Sciences, 1150, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, D. W. , Greer, F. R. , American Academy of Pediatrics Committee on Nutrition , & American Academy of Pediatrics Section on Gastroenterology, Hepatology, and Nutrition (2010). Probiotics and prebiotics in pediatrics. Pediatrics, 126(6), 1217–1231. [DOI] [PubMed] [Google Scholar]
- TRIGR Study Group (2007). Study design of the trial to reduce IDDM in the genetically at risk (TRIGR). Pediatric Diabetes, 8(3), 117–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNICEF . (2015). The baby‐friendly hospital initiative. Retrieved 9/9, 2015, from http://www.unicef.org/programme/breastfeeding/baby.htm
- Virtanen, S. M. , Hypponen, E. , Laara, E. , Vahasalo, P. , Kulmala, P. , Savola, K. , … Akerblom, H. K. (1998). Cow's milk consumption, disease‐associated autoantibodies and type 1 diabetes mellitus: A follow‐up study in siblings of diabetic children. Childhood diabetes in Finland study group. Diabetic Medicine: A Journal of the British Diabetic Association, 15(9), 730–738. [DOI] [PubMed] [Google Scholar]
- Virtanen, S. M. , Laara, E. , Hypponen, E. , Reijonen, H. , Rasanen, L. , Aro, A. , … Akerblom, H. K. (2000). Cow's milk consumption, HLA‐DQB1 genotype, and type 1 diabetes: A nested case‐control study of siblings of children with diabetes. childhood diabetes in finland study group. Diabetes, 49(6), 912–917. [DOI] [PubMed] [Google Scholar]
- Virtanen, S. M. , Nevalainen, J. , Kronberg‐Kippila, C. , Ahonen, S. , Tapanainen, H. , Uusitalo, L. , … Knip, M. (2012). Food consumption and advanced beta cell autoimmunity in young children with HLA‐conferred susceptibility to type 1 diabetes: A nested case‐control design. The American Journal of Clinical Nutrition, 95(2), 471–478. [DOI] [PubMed] [Google Scholar]
- Virtanen, S. M. , Rasanen, L. , Aro, A. , Lindstrom, J. , Sippola, H. , Lounamaa, R. , … Childhood Diabetes in Finland Study Group (1991). Infant feeding in Finnish children less than 7 yr of age with newly diagnosed IDDM. Childhood diabetes in Finland study group. Diabetes Care, 14(5), 415–417. [DOI] [PubMed] [Google Scholar]
- Virtanen, S. M. , Takkinen, H. M. , Nevalainen, J. , Kronberg‐Kippila, C. , Salmenhaara, M. , Uusitalo, L. , … Knip, M. (2011). Early introduction of root vegetables in infancy associated with advanced ss‐cell autoimmunity in young children with human leukocyte antigen‐conferred susceptibility to type 1 diabetes. Diabetic Medicine: A Journal of the British Diabetic Association, 28(8), 965–971. [DOI] [PubMed] [Google Scholar]
- von Berg, A. , Filipiak‐Pittroff, B. , Kramer, U. , Hoffmann, B. , Link, E. , Beckmann, C. , … GINIplus study group . (2013). Allergies in high‐risk schoolchildren after early intervention with cow's milk protein hydrolysates: 10‐Year results from the German infant nutritional intervention (GINI) study. The Journal of Allergy and Clinical Immunology, 131(6), 1565–1573. [DOI] [PubMed] [Google Scholar]
- von Berg, A. , Filipiak‐Pittroff, B. , Kramer, U. , Link, E. , Bollrath, C. , Brockow, I. , … GINIplus study group (2008). Preventive effect of hydrolyzed infant formulas persists until age 6 years: Long‐term results from the German infant nutritional intervention study (GINI). The Journal of Allergy and Clinical Immunology, 121(6), 1442–1447. [DOI] [PubMed] [Google Scholar]
- von Berg, A. , Koletzko, S. , Filipiak‐Pittroff, B. , Laubereau, B. , Grubl, A. , Wichmann, H. E. , … German Infant Nutritional Intervention Study Group (2007). Certain hydrolyzed formulas reduce the incidence of atopic dermatitis but not that of asthma: Three‐year results of the German infant nutritional intervention study. The Journal of Allergy and Clinical Immunology, 119(3), 718–725. [DOI] [PubMed] [Google Scholar]
- von Berg, A. , Koletzko, S. , Grubl, A. , Filipiak‐Pittroff, B. , Wichmann, H. E. , Bauer, C. P. , … German Infant Nutritional Intervention Study Group (2003). The effect of hydrolyzed cow's milk formula for allergy prevention in the first year of life: The German infant nutritional intervention study, a randomized double‐blind trial. The Journal of Allergy and Clinical Immunology, 111(3), 533–540. [DOI] [PubMed] [Google Scholar]
- Wahlberg, J. , Vaarala, O. , Ludvigsson, J. , & ABIS‐study group (2006). Dietary risk factors for the emergence of type 1 diabetes‐related autoantibodies in 21/2 year‐old swedish children. The British Journal of Nutrition, 95(3), 603–608. [DOI] [PubMed] [Google Scholar]
- World Health Organization . (2001). Nutrition‐complementary feeding. Retrieved 02/18, 2016, from http://www.who.int/nutrition/topics/complementary_feeding/en/
- World Health Organization . (2008). Indicators for assessing infant and young child feeding practices: Conclusions of a consensus meeting held 6–8 November 2007 in Washington D.C., USA. World Health Organization.
- World Health Organization . (2015). Health topics—Breastfeeding. Retrieved 9/9, 2015, from http://www.who.int/topics/breastfeeding/en/


