Abstract
Background: Although immune-related thyroiditis (irT) with immune checkpoint inhibitors (ICI) is a common consequence, its natural course and management recommendations are not well characterized in existing guidelines. This study sought to investigate the evolution of irT and describe its course and sequelae.
Methods: This was a retrospective study of cancer patients treated with ICI between November 2014 and July 2016 at MD Anderson Cancer Center and referred for endocrinology evaluation for suspected irT. Patients included had normal baseline thyroid function tests prior to starting ICI and developed thyrotoxicosis due to irT.
Results: Of 657 patients treated with ICI during the study period, 43(6.5%) met the inclusion criteria. ICI included: ipilimumab + nivolumab (40%), nivolumab (33%), pembrolizumab (21%), and other (7%). Cancer diagnoses observed were melanoma (23%), renal-cell carcinoma (21%), lung cancer (19%), bladder cancer (12%), colon cancer (9%), and other cancers (15%). Median time from ICI start to thyrotoxicosis was 5.3 weeks (range 0.6–19.6 weeks). Clinically, patients presented with painless thyroiditis, and 67% were asymptomatic during the thyrotoxicosis phase. Thyrotoxicosis lasted a median of six weeks (range 2.6–39.7 weeks). Hypothyroidism developed in 37 (84%) patients at a median of 10.4 weeks (range 3.4–48.7 weeks) after starting ICI. These patients remained on levothyroxine and ICI at a median follow-up of 57.4 weeks (range 1–156.7 weeks) from hypothyroidism onset. Four patients recovered without initiating levothyroxine and remained euthyroid at a median follow-up of 11.35 months (range 4.43–14.43 months). Subgroup analysis of ipilimumab + nivolumab versus nivolumab alone showed a median time to thyrotoxicosis of two weeks [confidence interval (CI) 3.5–8.4] versus six weeks ([CI 1.2–2.8]; p = 0.26) and time to hypothyroidism of 10 weeks [CI 8.1–11.9] versus 17 weeks ([CI 8.8–25.2]; p = 0.029) after starting ICI. Thyroid peroxidase and thyroglobulin antibodies were present in 45% and 33% at the time of irT diagnosis.
Conclusions: IrT manifests as an early onset of thyrotoxicosis, which is largely asymptomatic, followed by rapid transition to hypothyroidism requiring long-term levothyroxine substitution. The evolution of irT is more rapid with combination ICI. Frequent monitoring of thyroid function tests during ICI is warranted. Future guidelines need to recognize this entity and incorporate their management.
Keywords: : thyroiditis, immunotherapy, pembrolizumab, nivolumab, side effects
Introduction
Immune checkpoint inhibitors (ICIs) are cancer therapies that provide impressive clinical benefit in many advanced malignancies. These immunotherapies block the function of immune checkpoints, thereby promoting T cell–mediated antitumor responses. Optimal T-cell activation requires two signals. The first signal involves the interaction between the T-cell receptor (TCR) with its cognate peptide–major histocompatibility complex molecule expressed on antigen presenting cells (APCs). The second costimulatory signal comprises CD28, which is constitutively expressed on T cells, binding to B7 ligands expressed on professional APCs. Cytotoxic T lymphocyte antigen 4 (CTLA-4) is an immune checkpoint that bears structural similarity to the CD28, and it is upregulated on activated T cells and constitutively expressed on regulatory T cells. CTLA-4 competes with CD28 for binding to the B7 ligands, and it inhibits T cell–mediated immune responses (1). Other immune checkpoints belonging to the CD28/B7 superfamily have been identified, and they include programmed cell death protein 1 (PD-1) and its ligand, PD-L1. Monoclonal antibodies targeting CTLA-4 (ipilimumab, tremelimumab), PD-1 (nivolumab, pembrolizumab), and PD-L1 (durvalumab, atezolizumab, avelumab) have been designed to block the function of these immune checkpoint, resulting in enhanced antitumoral responses (2,3).
The limitations of ICIs include the development of a unique set of inflammatory side effects referred to as immune-related adverse events (irAEs). More commonly, they arise as a result of deregulated immune balance or immune equilibrium. IrAEs can affect any organ system, but they typically involve the skin and gastrointestinal (colon and liver) and endocrine systems (4). The most common endocrine irAEs include hypophysitis (with attendant hypopituitarism) and thyroid dysfunction (5). The reported prevalence of thyroid dysfunction varies greatly, ranging from 6% to 20% in large Phase III clinical trials of drugs targeting CTLA-4, PD-1, and PD-L1 (6–8). Thyroiditis is very infrequently reported as a sole irAE. In most studies examining ICI-mediated thyroiditis, a distinction is made between hypothyroidism, hyperthyroidism, and thyroiditis cases when in reality these are likely part of the same disease process. Several case series have attempted to characterize the clinical presentation, natural course, and pathophysiology of ICI-mediated thyroiditis (8–13). However, the small sample size and the lack of long-term follow-up precluded the development of specific recommendations for its management. With the rapidly expanding indication of the use of ICI, there is a pressing need to establish a standard of care in regards to the diagnosis, management, and long-term follow-up of ICI-mediated thyroiditis. The American Thyroid Association (ATA) guidelines describe several drugs responsible for drug-induced painless thyroiditis, such as amiodarone, lithium, interferon alfa, interleukin (IL)-2, and tyrosine kinase inhibitors (14). However, ICI is not included as a potential cause. In this study, which represents the largest cohort of patients with ICI-mediated thyroiditis to the authors' knowledge, the natural course of ICI-mediated thyroiditis is described, with the goal of providing guidelines for diagnosis and management.
Methods
Study population
Under an Institutional Review Board–approved protocol, medical records were retrospectively reviewed of patients on ICI treated at The University of Texas MD Anderson Cancer Center and referred for endocrinology evaluation of abnormal thyroid function tests (TFTs) between November 2014 and July 2016. In order to be studied further, patients had to meet the following inclusion criteria: the absence of preexisting thyroid disease, normal baseline TFTs within one year prior of starting ICI, and the presence of documented thyrotoxicosis due to thyroiditis. Patients who were hypothyroid without a documented thyrotoxic phase were excluded, with the goal of being able to describe the entire thyroiditis course. Patients who developed thyrotoxicosis from Graves' disease or toxic nodular goiter were excluded.
Definitions
Thyrotoxicosis was defined by a low serum thyrotropin (TSH) in the presence of a high or a normal free thyroxine (fT4) level. Peak fT4 was defined as the highest level of fT4 during ICI. Hypothyroidism was defined by the presence of either: (i) a low fT4, regardless of TSH, which occurred after thyrotoxicosis; or (ii) a high TSH (above the upper limit of normal), with a low or normal fT4. Reference ranges in the center's laboratory are 0.27–4.2 μIU/mL for TSH and 0.93–1.7 ng/dL for fT4.
The time to thyrotoxicosis was defined as the time from ICI start to the first documented laboratory evaluation consistent with thyrotoxicosis. The time to hypothyroidism was defined as the time from ICI start to the first documented laboratory evaluation consistent with hypothyroidism. The thyrotoxicosic phase was defined as the time from the first documented thyrotoxicosis event to the time of hypothyroidism. The hypothyroid phase was defined as the time from first documented hypothyroidism to last follow-up.
Outcomes
The primary outcome was to describe the evolution of thyroiditis with respect to its clinical presentation, time to thyrotoxicosis, time to hypothyroidism, and duration of each phase. Subgroup analyses were done for individual drugs to compare the timeline of thyroiditis among them to see if there was any difference in its evolution and presentation.
Statistics
IBM SPSS Statistics for Windows v21 (IBM Corp., Armonk, NY) was used to obtain Kaplan–Meier curves to describe the time to thyrotoxicosis and time to hypothyroidism. Descriptive statistics were used to report duration of thyrotoxic and hypothyroid phases, as well as laboratory values and dosages of levothyroxine replacement.
Results
Study population
A total of 657 patients were treated with an ICI during the study period. Of these, 56 (8.5%) patients were referred to endocrinology for evaluation of thyroid dysfunction potentially related to ICI therapy. Thirteen patients were excluded due to pre-existing hypothyroidism on levothyroxine replacement (n = 2), baseline TFTs more than a year before starting immunotherapy (n = 1), pre-existing Graves' disease (n = 1), hypothyroidism without documented thyrotoxicosis (n = 4), Graves' disease while on anti-CTLA-4 therapy (n = 2), and thyrotoxicosis due to co-existing toxic nodules (n = 3).
Forty-three (6.5%) patients were included in the final analysis. The baseline characteristics of the study population are described in Table 1. The median age at ICI start was 57 years (range 21–81 years). There was a similar representation of men and women, and 84% were Caucasian. The most common cancer diagnoses were melanoma (23%) and renal-cell carcinoma (21%), followed by lung cancer (19%), bladder cancer (12%), colon cancer (9%), central nervous system tumors (7%), ovarian cancer (4.5%), pancreatic cancer (2.25%), and non-Hodgkin's lymphoma (2.25%). Combination therapy with ipilimumab and nivolumab was the most commonly used ICI regimen in 17 (39.5%) patients followed by single-agent nivolumab in 14 (32.5%) patients and single-agent pembrolizumab in nine (21%) patients. Patients were followed for a median of 17.6 months (range 1–41.3 months) from the initiation of ICI. At the time of the data analysis, eight patients were still receiving ICI therapy.
Table 1.
Baseline Characteristics
| Patient characteristics | n = 43 |
|---|---|
| Age (years) at treatment start, median (range) | 57 (21–81) |
| Sex, n (%) | |
| Male | 22 (51) |
| Female | 21 (49) |
| Ethnicity, n (%) | |
| Caucasian | 36 (84) |
| Black | 3 (6) |
| Hispanic | 2 (5) |
| Asian | 2 (5) |
| Type of cancer, n (%) | |
| Melanoma | 10 (23) |
| Renal-cell carcinoma | 9 (21) |
| Lung cancer | 8 (19) |
| Urothelial carcinoma | 5 (12) |
| Colon cancer | 4 (9) |
| Glioblastoma multiforme | 3 (7) |
| Ovarian cancer | 2 (4.5) |
| Othersa | 2 (4.5) |
| Type of immunotherapy, n (%) | |
| Ipilimumab + nivolumab | 17 (39.5) |
| Nivolumab | 14 (32.5) |
| Pembrolizumab | 9 (21) |
| Durvalumab + tremelimumab | 2 (4.65) |
| Tremelimumab | 1 (2.35) |
| Follow-up time (months), median (range) | 17.63 (1–41.25) |
Other cancer diagnoses included pancreatic adenocarcinoma in one patient and non-Hodgkin's lymphoma in one patient.
Evolution of thyroiditis
Thyroid hormone levels
All 43 patients had normal TFTs drawn at baseline before starting ICI. The median time between baseline TFTs and the start of ICI was one day (range 0–269 days). At the time of thyrotoxicosis diagnosis, the median fT4 was 2.46 ng/dL (range 1.2–6.32 ng/dL), and the median TSH was 0.02 μIU/mL (range 0.01–0.23 μIU/mL). All patients had repeat laboratory testing at a median of 3.3 weeks (range 0.3–12.5 weeks) after the onset of the thyrotoxicosis phase, and 18 had an upward trend in fT4 to a median peak fT4 of 3.76 ng/dL (range 2.07 to >7.77 ng/dL). Hypothyroidism was marked by the presence of a median fT4 of 0.74 ng/dL (range 0.34–1.72 ng/dL) and a median TSH of 7.93 μIU/mL (range 0.37–168 μIU/mL).
Timeline of thyroiditis
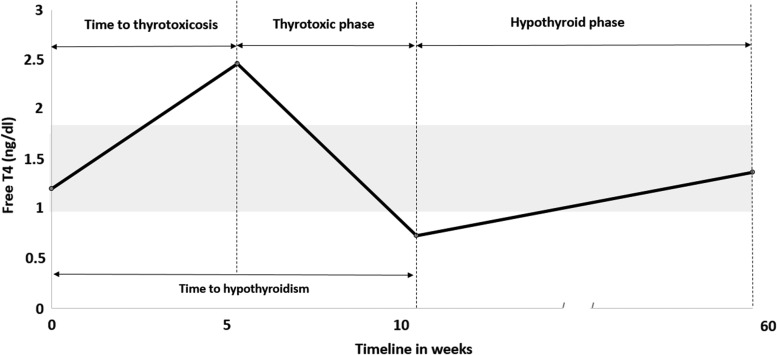
The thyroiditis timeline is described in Figure 1. Median time to thyrotoxicosis was 5.3 weeks (range 0.57–19.57 weeks). Median time to hypothyroidism was 10.4 weeks (range 3.4–48.71 weeks). The median thyrotoxicosis phase was six weeks (2.6–39.7 weeks). Thyrotoxicosis was seen after a median of two ICI doses (range 1–12 doses). In the patients whose peak fT4 levels were available, this peak occurred a median of 13 days (range 2–28 days) after the onset of first documented thyrotoxicosis. Of 43 patients, 37 (84%) developed hypothyroidism, and they were subsequently started on thyroid hormone replacement. Four (9%) patients recovered from transient hypothyroidism without requiring levothyroxine, and two patients died before they could develop hypothyroidism. The median hypothyroid phase was 57.4 weeks (range 1–156.7 weeks). At the time of hypothyroidism, four patients had both a low fT4 and low TSH that occurred after the thyrotoxic phase. None of these patients had clinical suspicion of central hypothyroidism. Of these, three were started on thyroid hormone before their TSH rose. In one patient, thyroid hormone was started when the TSH rose to 34 μIU/mL, two weeks after the first documented low fT4. Four patients (two on nivolumab, one on pembrolizumab, and one on tremelimumab) were initially monitored with observation for mild hypothyroidism (TSH of 4.45 μIU/mL and 4.8 μIU/mL with normal fT4 in two patients, TSH of 4.7 μIU/mL with fT4 of 0.86 ng/dL in one patient, and fT4 of 0.86 ng/dL with a normal TSH of 1.13 μIU/mL in one patient) recovered thyroid function at 15, 6, 5, and 4 weeks, respectively, without starting levothyroxine. They remain euthyroid at a median follow-up of 11.35 months (range 4.43–14.43 months) from the date of diagnosis of hypothyroidism. Two patients died from their cancer before becoming hypothyroid. All 37 hypothyroid patients remained on levothyroxine at a median follow-up time of 17.63 months (range 1–41.4 months) from the start of ICI. The median dose of levothyroxine required to achieve euthyroid status was 1.2 μg/kg (range 0.25–3 μg/kg). Ten (28%) patients required a final levothyroxine dose of <1 μg/kg, 17 (47%) patients between 1 and 1.6 μg/kg, and nine (25%) patients >1.6 μg/kg to remain euthyroid. At the time of last follow-up, the median TSH was 1.81 μIU/mL (range 0.07–4.09 μIU/mL) and the median fT4 was 1.38 ng/dL (range 1–1.8 ng/dL). Due to other irAEs, 21 patients received doses of steroids >0.5 mg/kg of prednisone or its equivalent during the course of thyroiditis. Of these, four (19%) patients also developed hypophysitis in addition to thyroiditis. Table 2 describes the effect of steroid exposure during ICI therapy on the dose of levothyroxine. All four patients who recovered thyroid function without thyroid hormone supplementation were exposed to steroids for other irAEs during the time of the irT event: dexamethasone 2–4 mg as a part of chemotherapy regimen in two patients, intravenous (i.v.) methylprednisolone 1 mg/kg q12H for colitis in one patient, and physiological hydrocortisone replacement in one patient who also had concomitant hypophysitis.
FIG. 1.
Timeline of thyroiditis. The graph depicts the timeline of thyroiditis from the start of ICI until the last follow up. Median time to thyrotoxicosis was 5.3 weeks (range 0.57–19.57 weeks). Median time to hypothyroidism was 10.4 weeks (range 3.4–48.71 weeks). Median thyrotoxicosis phase was 6 weeks (2.6–39.7 weeks). Median hypothyroid phase was 57.4 weeks (range 1–156.7 weeks). There was no recovery in thyroid function seen in the patients who were started on levothyroxine, when followed until the date of last follow up at the time of data analysis. ICI, immune checkpoint inhibitors.
Table 2.
Effect of Steroid Exposure and Elevated Thyroid Antibodies on Levothyroxine Dose
| Antibody and steroid exposure versus dose | <1 μg/kg (n/10) | 1–1.6 μg/kg (n/17) | >1.6 μg/kg (n/9) |
|---|---|---|---|
| Ab+/steroid+, (n) | 1 | 4 | 0 |
| Ab+/steroid−, (n) | 1 | 4 | 4 |
| Ab−/steroid+, (n) | 6 | 3 | 2 |
| Ab−/steroid−, (n) | 2 | 3 | 1 |
Any steroid exposure either intravenous or oral physiological or supraphysiological, anytime during the course of thyroiditis was documented as positive. Antibodies (Ab) detected were antithyroid peroxidase antibodies and/or antithyroglobulin antibodies.
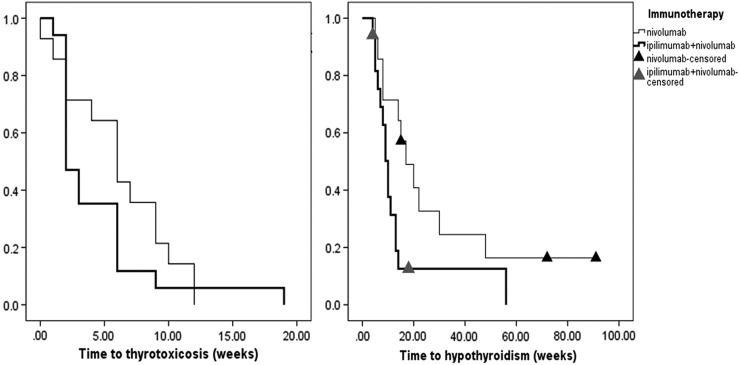
A subgroup analysis describing the timeline differences based on type of therapy is shown in Table 3 and Figure 2. Figure 2 depicts the differences between combination nivolumab with ipilimumab versus single-agent nivolumab. Median time to thyrotoxicosis was two weeks [CI 1.19–2.8] with the combination versus six weeks with nivolumab alone ([CI 3.6–8.4], p = 0.26). The median frequency of laboratory evaluation from the initiation of ICI to the onset of thyrotoxicosis was of 0.35 per week in the former group versus 0.41 per week in the latter group (p = 0.82). The duration of the thyrotoxic phase with the combination of ipilimumab and nivolumab was a median of six weeks [CI 4.07–7.92] versus a median of 10 weeks [CI 8.18–11.81] with nivolumab. During this phase, the frequency of laboratory evaluation was 0.35 per week in the former group versus 0.35 evaluations per week in the latter group (p = 0.12). The time to hypothyroidism was 10 weeks with the combination of ipilimumab and nivolumab [CI 8.1–11.9] versus 17 weeks [CI 8.82–25.18] with nivolumab alone (p = 0.029). With pembrolizumab, the median time to thyrotoxicosis from the start of ICI was five weeks [CI 3.15–6.84], and the thyrotoxic phase lasted a median of five weeks [CI 3.61–6.38]. Hypothyroidism developed at median of 10 weeks [CI 8.61–11.38] after starting pembrolizumab. In the two patients treated with durvalumab and tremelimumab, the median time to thyrotoxicosis from the start of ICI was six weeks (range 6–6.14 weeks), and the thyrotoxic phase lasted a median of nine weeks (range 6–12 weeks). Hypothyroidism developed at a median of 15 weeks (range 12–18 weeks) after starting the ICI. In the patient who received tremelimumab, thyrotoxicosis developed five weeks after starting ICI. This patient developed transient hypothyroidism with fT4 of 0.86 ng/dL without elevation of TSH, which soon improved without any evidence of hypothyroidism after six months of follow-up.
Table 3.
Timeline of Thyroiditis Depending on Individual Drug Regimen
| Timeline (weeks) | Pembrolizumab (n = 9) | Nivolumab (n = 14) | Ipilimumab + nivolumab (n = 17) | p |
|---|---|---|---|---|
| Time to thyrotoxicosis | 5 [3.15–6.84] | 6 [3.58–8.41] | 2 [1.19–2.8] | 0.423 |
| Thyrotoxicosis phase | 5 [3.61–6.38] | 10 [8.18–11.81] | 6 [4.07–7.92] | 0.05 |
| Time to hypothyroidism | 10 [8.61–11.38] | 17 [8.82–25.18] | 10 [8.11–11.90] | 0.09 |
Data shown are median [confidence interval].
FIG. 2.
Comparison of timeline of thyroiditis with nivolumab versus combination of ipilimumab + nivolumab. The time to thyrotoxicosis with nivolumab was six weeks [confidence interval (CI) 3.6–8.4] versus two weeks [CI 1.19–2.8] with the combination of ipilimumab + nivolumab (p = 0.26). The time to hypothyroidism from the start of ICI was 10 weeks with the combination of ipilimumab and nivolumab [CI 8.1–11.9] versus 17 weeks [CI 8.82–25.18] with nivolumab alone (p = 0.029). Two patients on ipilimumab and nivolumab died prior to developing hypothyroidism.
Clinical presentation
All patients experienced painless thyroiditis. During the thyrotoxic phase, 14/43 patients (33%) were symptomatic, with palpitations being the most common symptom, followed by tremors, heat intolerance, weight loss, and fatigue. Among the individual therapies, 7/9 (78%) patients in the pembrolizumab group, 5/17 (29%) patients in the ipilimumab + nivolumab group, and 2/14 (14%) patients in the nivolumab group experienced symptoms of thyrotoxicosis. Two patients—one patient on pembrolizumab and one patient on a combination of ipilimumab and nivolumab—developed atrial fibrillation with rapid ventricular rate, which required holding immunotherapy. However, ICI treatment was not discontinued in any patient. For all symptomatic patients, conservative management with beta blockers was sufficient. Five (12%) patients were symptomatic during the hypothyroid phase, with weight gain, constipation, excessive hair loss, and/or cold intolerance or fatigue.
Thyroid autoantibody testing and imaging
Baseline autoimmune thyroid status was not known in any of these patients, as it is not standard care to evaluate for this in patients with normal thyroid function. During the thyrotoxic phase, serologic tests were performed in 38 patients and imaging in 11 patients. Out of 38 patients tested, 17 (44.7%) had elevated thyroid peroxidase (TPO) antibodies (>35 IU/mL), and of 21 tested, nine (33%) had elevated thyroglobulin antibodies (>40 IU/mL). Thirty-two patients were checked for thyroid-stimulating immunoglobulins or thyrotropin receptor antibodies, and all were negative. Anti-TPO antibodies were present in 8/15 (53%) patients in the nivolumab + ipilimumab group, 4/14 (29%) patients in the nivolumab group, and 1/7 (14.3%) patients in the pembrolizumab group. The presence of these antibodies did not have a statistical significant impact on the evolution timeline of thyroiditis.
Of the 11 patients who underwent thyroid ultrasound, 7 (62%) exhibited heterogeneity and low vascularity consistent with thyroiditis. Of the 43 patients, 7 (16%) had nuclear imaging. All of them revealed low uptake consistent with thyroiditis.
Discussion
The natural course of ICI-mediated thyroiditis was studied in a large cohort of cancer patients receiving different immunotherapies. The results demonstrate that ICI induces a rapidly destructive phenomenon comprising a largely asymptomatic thyrotoxic phase followed by precipitous transition to hypothyroidism that is likely permanent. ICI-mediated thyroiditis has not yet been included as an etiology in the existing hyperthyroidism and thyrotoxicosis guidelines (14). Compared to other drugs, treatment with ICI results in a different thyroiditis course that should be approached differently. Drugs commonly recognized as being associated with thyroiditis are amiodarone, lithium, interferon alfa, IL-2, and tyrosine kinase inhibitors. Painless thyroiditis described in the literature encompasses a thyrotoxic phase in a minority of patients (5–20%) followed by a hypothyroid phase that lasts around six months, and there is recovery of normal function in most patients, with about 10–20% developing permanent hypothyroidism (14).
The true prevalence of ICI-mediated thyroiditis is not clearly reported and is likely under-reported. ICI-mediated hyperthyroidism and hypothyroidism have been reported as individual events with anti-CTLA-4 and anti-PD-1 therapy, but the natural course of destructive thyroiditis has not yet been described (15,16). European studies have reported hyperthyroid events ranging from 1% to 10% and hypothyroid events ranging from 1% to 15% in cancer patients treated with pembrolizumab or with nivolumab alone or combined with ipilimumab (16). The present study found that 6.5% of patients receiving ICI and referred to endocrinology were diagnosed with ICI-mediated thyroiditis, suggesting a prevalence of 6.5% or more if one takes into account the cases not referred for endocrine evaluation. Smaller case series have reported ICI-mediated thyroiditis in patients with melanoma and non–small cell lung cancer who were treated with either anti-CTLA4 or anti-PD1 therapy (8,12,17). Very few cases of thyroiditis have been described as a separate entity. In a large institutional case series of endocrine irAEs in patients on ipilimumab for the treatment of advanced melanoma, 6/246 patients developed thyroiditis followed by hypothyroidism (8). Two separate case series of patients on anti-PD1 therapy have described six and three patients, respectively, who developed thyroiditis followed by hypothyroidism requiring hormone replacement (11,12). In another study, 17/99 patients with advanced melanoma who were started on pembrolizumab developed thyroid AEs. Of these, nine patients progressed to hypothyroidism from thyrotoxicosis, suggesting that these might be parts of the spectrum of thyroiditis (10). Another study described 13 patients with pembrolizumab-induced thyroiditis, wherein three patients developed hypothyroidism, four patients recovered thyroid function, and six remained thyrotoxic at the time of last follow-up (9).
This study found that ICI-mediated thyroiditis appears to be more commonly associated with anti-PD1 drugs used alone or in combination with anti-CTLA-4 agents. About 40% of patients who developed thyroiditis were on a combination of anti-CTLA-4 and anti-PD-1 therapy. No patient developed thyroiditis leading to permanent hypothyroidism on anti-CTLA-4 monotherapy. This finding is consistent with previously reported higher prevalence of thyroid dysfunction in patients receiving anti PD-1 agents alone or combined with anti-CTLA-4 agents in large clinical trials (18).
In the present cohort, first documentation of thyrotoxicosis was observed at an average of five weeks after starting ICI, but also occurred as early as four days or as late as 20 weeks from initiation of ICI. More than 40% of patients had an upward trend in fT4 levels, with the next laboratory evaluation occurring at a median of two weeks after the onset of first documented thyrotoxicosis. This finding may be due to a more proactive hormonal evaluation in these patients. Hypothyroidism occurred at a median of six weeks from the first documented thyrotoxicosis event and 10 weeks after the first dose of ICI. There was no statistically significant difference in the timeline of thyroiditis when comparing all individual therapies. However, the time to thyrotoxicosis, albeit not statistically significant, was shorter in the group receiving combination therapy with ipilimumab and nivolumab (two weeks) compared to nivolumab alone (six weeks). Additionally, significantly more rapid onset of hypothyroidism was found with combined ipilimumab and nivolumab (10 weeks) than with single-agent nivolumab (17 weeks). The frequency of TFT monitoring was similar in both groups, suggesting that the combination is likely to cause a more abrupt clinical course. The time to thyrotoxicosis with pembrolizumab (five weeks) was similar to that reported earlier (9,10). While the timeline of thyroiditis with combination of ipilimumab and nivolumab has not been previously described, the timeline for the onset of the thyrotoxic phase with single-agent nivolumab in this study was more indolent at a median of six weeks compared to the three weeks reported in other studies (12,13). The patients in the present study were followed for a prolonged time after development of hypothyroidism (>14 months), and all patients who were started on levothyroxine remained on thyroid hormone replacement with normal TFTs at their last follow-up. These results suggest that hypothyroidism may require lifelong treatment. The median dose of levothyroxine was 1.2 μg/kg, lower than 1.6 μg/kg daily, which is the usual replacement dose of levothyroxine for those with surgical hypothyroidism for nonthyroidal cancer-related causes (19). While the effect of high-dose steroid exposure on thyroiditis course was not studied, patients who were exposed to glucocorticoids during the course of thyroiditis were observed to require lower doses of levothyroxine than those who did not. Only a small proportion of patients (4/43) did not develop hypothyroidism requiring levothyroxine replacement. All four of these patients who recovered normal thyroid function after thyrotoxicosis received courses of high-dose systemic glucocorticoids either as a part of chemotherapy regimen or for other irAEs at some point in their thyroiditis course. The majority of patients experienced painless thyroiditis and were asymptomatic. In the 14 (33%) patients who were symptomatic during the thyrotoxic phase, the commonly encountered symptoms of palpitations and tremors were managed conservatively with beta-blockers, and all patients were able to continue ICI. The fact that more patients in the combination group were symptomatic with thyrotoxicosis than with single-agent nivolumab is consistent with the results of the melanoma study, which reported that the incidence of grade 3 or 4 events was 54% in the combination group versus 24% in the nivolumab group alone (20). Interestingly, the majority of patients on pembrolizumab in the present study were symptomatic during the thyrotoxicosis phase. The results are different from the existing literature, which reported that most patients are largely asymptomatic (9,10). Those patients who underwent nuclear imaging studies showed low uptake of iodine or 99mTc pertechnetate, suggesting a destructive thyroiditis.
The role of antibodies in the pathophysiology of immune-related thyroiditis has not yet been prospectively studied or elucidated. Autoimmunity is believed to play a role in the pathogenesis of other causes of painless thyroiditis, with about 50% of patients having anti-TPO antibodies (14). The frequency of antibody positivity at the time of immune-related thyroiditis diagnosis in this study is similar to what has been previously reported. As described in the literature, patients with pre-existing thyroid autoimmunity were more at risk of developing thyroiditis with use of interleukin, amiodarone, and lithium (14). Though antibodies were measured in most patients in this study, it remains unclear if they had a role in the development of thyroiditis or eventual hypothyroidism due to lack of baseline values. However, it was observed that the patients who had elevated anti-TPO or antithyroglobulin antibodies required a higher dose of levothyroxine than those who did not have elevated antibodies. The higher prevalence antibody positivity in the combination group with ipilimumab and nivolumab (53%) versus nivolumab alone (29%) may suggest a more robust immune response in the former, leading to a faster destruction of the thyroid. As described above, the combination ICI therapy resulted in a more rapid thyroiditis evolution, and more patients were symptomatic compared to monotherapy. In one reported study, the mechanism of pembrolizumab-induced thyroiditis was thought to be mediated by circulating CD56, CD16, and natural killer cells, a different phenotype from autoimmune thyroid disease (9). Another study described the association of PD-L1 and PD-L2 expression on thyroid surface and destructive thyroiditis (13). Other studies have described the worsening of pre-existing Hashimoto's disease and subclinical hypothyroidism in patients treated with immunotherapy (13,21). These reports suggest possible synergistic roles of autoimmune and inflammatory mechanisms in the destruction of the thyroid gland, which needs to be evaluated further. Furthermore, some studies have described the role of CTLA-4 and its polymorphisms in the development of autoimmune thyroid disease (22,23). ICI-mediated Graves' disease has been reported in the literature (24,25). Testing for antibodies supported by appropriate imaging might help diagnose ICI-mediated Graves' disease, for which the management is different using antithyroid medications or radioactive iodine (4,25).
Existing guidelines recommend that thyroid hormone levels be checked at least every six months for patients with drug-induced thyroiditis (14). However, based on this study, it is concluded that checking at six-month intervals would miss not just the thyrotoxic phase but also the onset of hypothyroidism in patients receiving ICI, potentially leading to worsening patient morbidity associated with untreated hypothyroidism. More frequent testing, either prior to each dose of ICI or at two- to three-week intervals, will help facilitate timely intervention. Although rare, coexisting endocrinopathies such as hypophysitis leading to central adrenal insufficiency and central hypothyroidism can occur, making the diagnosis challenging. In agreement with the current findings, more recent oncology guidelines recommend routine thyroid function monitoring before each dose for all patients treated with ICI (26,27). For patients who develop thyrotoxicosis, close monitoring with regular symptom evaluation and fT4 testing every 2 weeks is recommended. Once patients develop hypothyroidism (low fT4 after a documented thyrotoxic phase, even if the TSH is not elevated), initiation of thyroid hormone replacement per standard guidelines is recommended (26). The goal of this study was to focus on patients with both a thyrotoxicosis and hypothyroid phase in order to describe the entire thyroiditis course. It is acknowledged that there are patients who may develop primary hypothyroidism without a documented thyrotoxic phase. This can occur for two reasons: (i) the thyrotoxic phase was missed due to its rapid asymptomatic evolution, or (ii) patients develop hypothyroidism without a thyrotoxicosis phase.
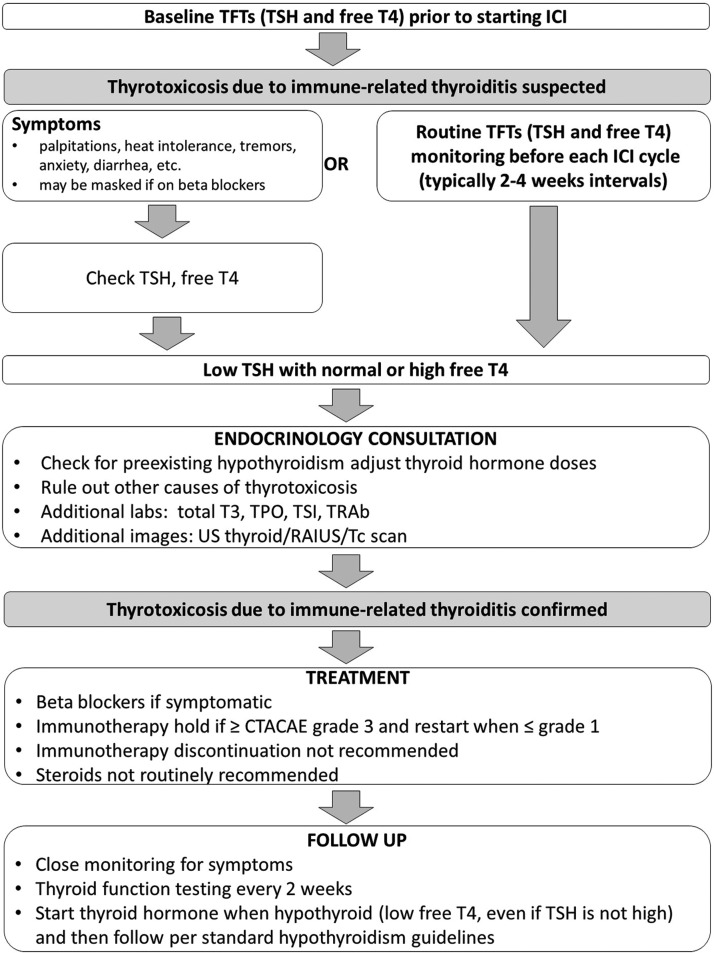
A proposed algorithm for the evaluation and treatment of ICI-mediated thyroiditis is shown in Figure 3.
FIG. 3.
Proposed algorithm for management of immune checkpoint inhibitor (ICI)-mediated thyroiditis. The figure below outlines a proposed algorithm for evaluation in patient on ICI therapy in whom thyroiditis is suspected. Common Terminology Criteria for Adverse Events (CTCAE) v.4 criteria: grade 1—asymptomatic; clinical or diagnostic observations only; intervention not indicated; grade 2—symptomatic; thyroid suppression therapy indicated; limiting instrumental activities of daily living (ADL); grade 3: severe symptoms limiting self-care ADL and hospitalization indicated; grade 4—life-threatening consequences; urgent intervention indicated; grade 5: death.
Due to the retrospective nature of the study, its limitations are related to possible referral bias. As no patients were taken off thyroid hormone at the time of last follow-up, recovery cannot be clearly assessed, and therefore the duration of the hypothyroid phase is simply dependent upon follow-up. The retrospective analysis and limited samples size mean that strong inferences regarding the roles of autoimmunity or steroids in the natural history of thyroiditis cannot be made. Prospective large-scale studies are needed to evaluate these questions further.
Conclusion
With the rapidly expanding indication for the use of ICI in cancer, the prevalence of ICI-mediated thyroiditis of at least 6.5% translates into a new endocrine abnormality that affects many patients. ICI-mediated thyroiditis is characterized by the rapid development of a predominantly asymptomatic thyrotoxic phase followed by a quick transition to hypothyroidism that may require lifelong thyroid hormone replacement. The evolution of thyroiditis appears to be more rapid in patients treated with combination anti-CTLA-4 and anti-PD1 therapy. Early and frequent monitoring of TFTs in patients on ICI is warranted. The role of antibodies in the pathophysiology of immune-related thyroiditis is yet to be elucidated.
Acknowledgments
This work was supported by the NIH/NCI under award number P30CA016672.
Author Disclosure Statement
R.D. is a member of the Bristol Myers Squibb advisory board. The remaining authors have nothing to disclose.
References
- 1.Walker LS, Sansom DM. 2011. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat Rev Immunol 11:852–863 [DOI] [PubMed] [Google Scholar]
- 2.Kong YC, Flynn JC. 2014. Opportunistic autoimmune disorders potentiated by immune-checkpoint inhibitors anti-CTLA-4 and anti-PD-1. Front Immunol 5:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fife BT, Bluestone JA. 2008. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev 224:166–182 [DOI] [PubMed] [Google Scholar]
- 4.Dadu R, Zobniw C, Diab A. 2016. Managing adverse events with immune checkpoint agents. Cancer J 22:121–129 [DOI] [PubMed] [Google Scholar]
- 5.Boutros C, Tarhini A, Routier E, Lambotte O, Ladurie FL, Carbonnel F, Izzeddine H, Marabelle A, Champiat S, Berdelou A, Lanoy E, Texier M, Libenciuc C, Eggermont AM, Soria JC, Mateus C, Robert C. 2016. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol 13:473–486 [DOI] [PubMed] [Google Scholar]
- 6.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. 2010. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob J, Cowey CL, Lao CD, Schadendorf D, Ferrucci PF, Smylie M, Dummer R, Hill A, Haanen J, Maio M, McArthur G, Yang A, Rollin L, Horak C, Larkin J, Hodi FS. 2015. CheckMate 067: a Phase III randomized double-blind study of nivolumab (NIVO) monotherapy or NIVO combined with ipilimumab (IPI) versus IPI monotherapy in previously untreated patients (pts) with advanced melanoma (MEL). Ann Oncol 26:28–28 [Google Scholar]
- 8.Ryder M, Callahan M, Postow MA, Wolchok J, Fagin JA. 2014. Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: a comprehensive retrospective review from a single institution. Endocr Relat Cancer 21:371–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delivanis DA, Gustafson MP, Bornschlegl S, Merten MM, Kottschade L, Withers S, Dietz AB, Ryder M. 2017. Pembrolizumab-induced thyroiditis: comprehensive clinical review and insights into underlying involved mechanisms. J Clin Endocrinol Metab 102:2770–2780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Filette J, Jansen Y, Schreuer M, Everaert H, Velkeniers B, Neyns B, Bravenboer B. 2016. Incidence of thyroid-related adverse events in melanoma patients treated with pembrolizumab. J Clin Endocrinol Metab 101:4431–4439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanaka R, Fujisawa Y, Maruyama H, Nakamura Y, Yoshino K, Ohtsuka M, Fujimoto M. 2016. Nivolumab-induced thyroid dysfunction. Jpn J Clin Oncol 46:575–579 [DOI] [PubMed] [Google Scholar]
- 12.Orlov S, Salari F, Kashat L, Walfish PG. 2015. Induction of painless thyroiditis in patients receiving programmed death 1 receptor immunotherapy for metastatic malignancies. J Clin Endocrinol Metab 100:1738–1741 [DOI] [PubMed] [Google Scholar]
- 13.Yamauchi I, Sakane Y, Fukuda Y, Fujii T, Taura D, Hirata M, Hirota K, Ueda Y, Kanai Y, Yamashita Y, Kondo E, Sone M, Yasoda A, Inagaki N. 2017. Clinical features of nivolumab-induced thyroiditis: a case series study. Thyroid 27:894–901 [DOI] [PubMed] [Google Scholar]
- 14.Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, Rivkees SA, Samuels M, Sosa JA, Stan MN, Walter MA. 2016. 2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid 26:1343–1421 [DOI] [PubMed] [Google Scholar]
- 15.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, Linette GP, Meyer N, Giguere JK, Agarwala SS, Shaheen M, Ernstoff MS, Minor D, Salama AK, Taylor M, Ott PA, Rollin LM, Horak C, Gagnier P, Wolchok JD, Hodi FS. 2015. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. New Engl J Med 372:2006–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spain L, Diem S, Larkin J. 2016. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev 44:51–60 [DOI] [PubMed] [Google Scholar]
- 17.Tanaka R, Fujisawa Y, Maruyama H, Nakamura Y, Yoshino K, Ohtsuka M, Fujimoto M. 2016. Nivolumab-induced thyroid dysfunction. Jpn J Clin Oncol 46:575–579 [DOI] [PubMed] [Google Scholar]
- 18.Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, Berdelou A, Varga A, Bahleda R, Hollebecque A, Massard C, Fuerea A, Ribrag V, Gazzah A, Armand JP, Amellal N, Angevin E, Noel N, Boutros C, Mateus C, Robert C, Soria JC, Marabelle A, Lambotte O. 2016. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer 54:139–148 [DOI] [PubMed] [Google Scholar]
- 19.Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, Mechanick JI, Pessah-Pollack R, Singer PA, Woeber KA; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults 2012. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract 18:988–1028 [DOI] [PubMed] [Google Scholar]
- 20.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, Linette GP, Meyer N, Giguere JK, Agarwala SS, Shaheen M, Ernstoff MS, Minor D, Salama AK, Taylor M, Ott PA, Rollin LM, Horak C, Gagnier P, Wolchok JD, Hodi FS. 2015. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 372:2006–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Narita T, Oiso N, Taketomo Y, Okahashi K, Yamauchi K, Sato M, Uchida S, Matsuda H, Kawada A. 2016. Serological aggravation of autoimmune thyroid disease in two cases receiving nivolumab. J Dermatol 43:210–214 [DOI] [PubMed] [Google Scholar]
- 22.Ueda H, Howson JM, Esposito L, Heward J, Snook H, Chamberlain G, Rainbow DB, Hunter KM, Smith AN, Di Genova G, Herr MH, Dahlman I, Payne F, Smyth D, Lowe C, Twells RC, Howlett S, Healy B, Nutland S, Rance HE, Everett V, Smink LJ, Lam AC, Cordell HJ, Walker NM, Bordin C, Hulme J, Motzo C, Cucca F, Hess JF, Metzker ML, Rogers J, Gregory S, Allahabadia A, Nithiyananthan R, Tuomilehto-Wolf E, Tuomilehto J, Bingley P, Gillespie KM, Undlien DE, Ronningen KS, Guja C, Ionescu-Tirgoviste C, Savage DA, Maxwell AP, Carson DJ, Patterson CC, Franklyn JA, Clayton DG, Peterson LB, Wicker LS, Todd JA, Gough SC. 2003. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature 423:506–511 [DOI] [PubMed] [Google Scholar]
- 23.Sanderson K, Scotland R, Lee P, Liu D, Groshen S, Snively J, Sian S, Nichol G, Davis T, Keler T, Yellin M, Weber J. 2005. Autoimmunity in a phase I trial of a fully human anti-cytotoxic T-lymphocyte antigen-4 monoclonal antibody with multiple melanoma peptides and Montanide ISA 51 for patients with resected stages III and IV melanoma. J Clin Oncol 23:741–750 [DOI] [PubMed] [Google Scholar]
- 24.Borodic G, Hinkle DM, Cia Y. 2011. Drug-induced graves disease from CTLA-4 receptor suppression. Ophthal Plast Reconstr Surg 27:e87–88 [DOI] [PubMed] [Google Scholar]
- 25.Azmat U, Liebner D, Joehlin-Price A, Agrawal A, Nabhan F. 2016. Treatment of ipilimumab induced Graves' disease in a patient with metastatic melanoma. Case Rep Endocrinol 2016:2087525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puzanov I, Diab A, Abdallah K, Bingham CO 3rd, Brogdon C, Dadu R, Hamad L, Kim S, Lacouture ME, LeBoeuf NR, Lenihan D, Onofrei C, Shannon V, Sharma R, Silk AW, Skondra D, Suarez-Almazor ME, Wang Y, Wiley K, Kaufman HL, Ernstoff MS; Society for Immunotherapy of Cancer Toxicity Management Working Group 2017. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer 5:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner JM, Ginex P, Hallmeyer S, Holter Chakrabarty J, Leighl NB, Mammen JS, McDermott DF, Naing A, Nastoupil LJ, Phillips T, Porter LD, Puzanov I, Reichner CA, Santomasso BD, Seigel C, Spira A, Suarez-Almazor ME, Wang Y, Weber JS, Wolchok JD, Thompson JA; National Comprehensive Cancer Network 2018. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 36:1714–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]