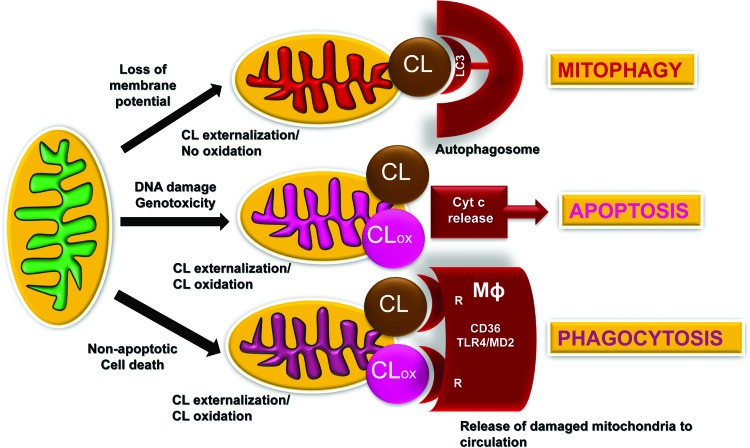
FIG. 2.
Schema illustrating the signaling mechanisms for externalized and oxidized CL in mitochondria, extra-mitochondrial and extra-cellular compartments. Damage to mitochondria resulting in IMM depolarization triggers the process of CL externalization to the mitochondrial surface whereby CL is recognized by one of the major components of the autophageal machinery, LC3. This process of CL redistribution from the IMM to the OMM and binding of CL with LC3 initiates mitophagy of injured mitochondria, and it is important for the mitochondrial quality control. Successful removal of damaged mitochondria serves a pro-survival function and maintains normal cell metabolism. If mitochondrial injury exceeds the reparative capacities of a given cell (e.g., excessive DNA damage), redistribution of CLs to the OMM creates conditions for its interaction with an intermembrane space hemoprotein, cyt c to yield a complex with a peroxidase catalytic competence toward PUFA-CL. CLox acts as a pro-apoptotic signal facilitating release of cyt c from mitochondria into the cytosol, thus designating a point of no-return in the execution of the intrinsic apoptotic program. Damaged mitochondria and/or their fragments with externalized CL and CLox are recognized by CD36-driven mechanisms of professional phagocytes, thus leading to their elimination by macrophages. Externalized CL (and CLox) may also interact with the MD2/TLR4 system on the surface of macrophages, leading to silencing of cytokine production and immune-paralysis. CL, cardiolipin; CLox, oxidized CL; IMM, inner mitochondrial membranes; LC3, light chain 3; MD2, myeloid differentiation protein 2; OMM, outer mitochondrial membrane; PUFA, polyunsaturated fatty acids; TLR4, toll-like receptor 4. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars