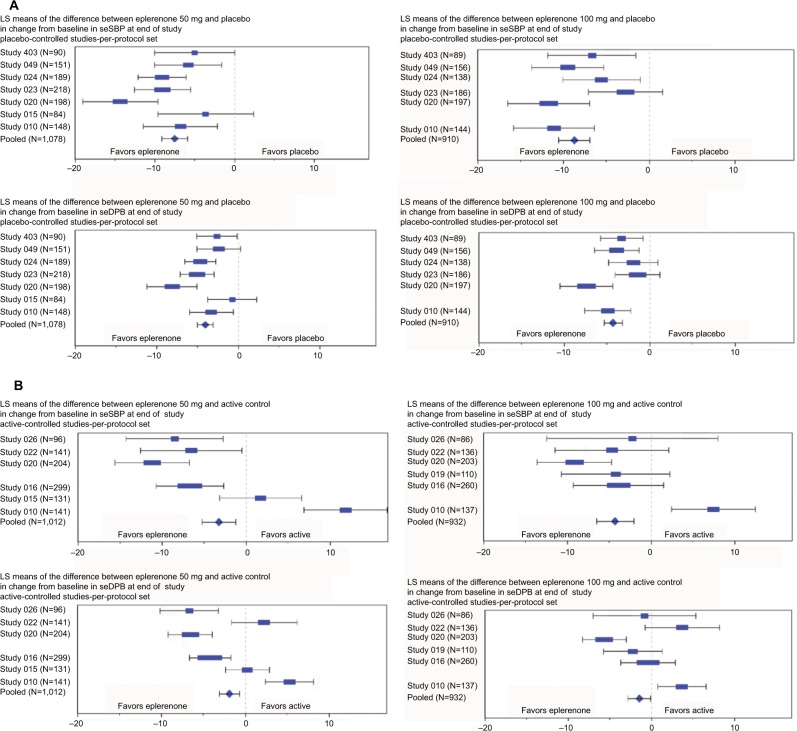
Figure 2.
Change from baseline in seated SBP/DBP: (A) placebo-controlled studies and (B) active-controlled studies.
Notes: LS Means model includes Study, Treatment, and Baseline BP. The per-protocol analysis set included all patients who were randomly assigned to treatment (eplerenone 50 mg daily, eplerenone 100 mg daily, or active control), who had a baseline primary efficacy evaluation, took at least one dose of study drug, and received at least 6 weeks of fixed dose of study drug. Weight of blue symbol is defined as percentage study contribution to total pooled results. Study 015 is not represented in the eplerenone 100 mg analysis due to lack of treatment arm with that dosage. Study 019 is not represented in the eplerenone 50mg analysis due to lack of treatment arm with that dosage. See Table 3 for the 95% confidence intervals of differences in seated SBP/DBP between eplerenone and placebo.