Figure 3.
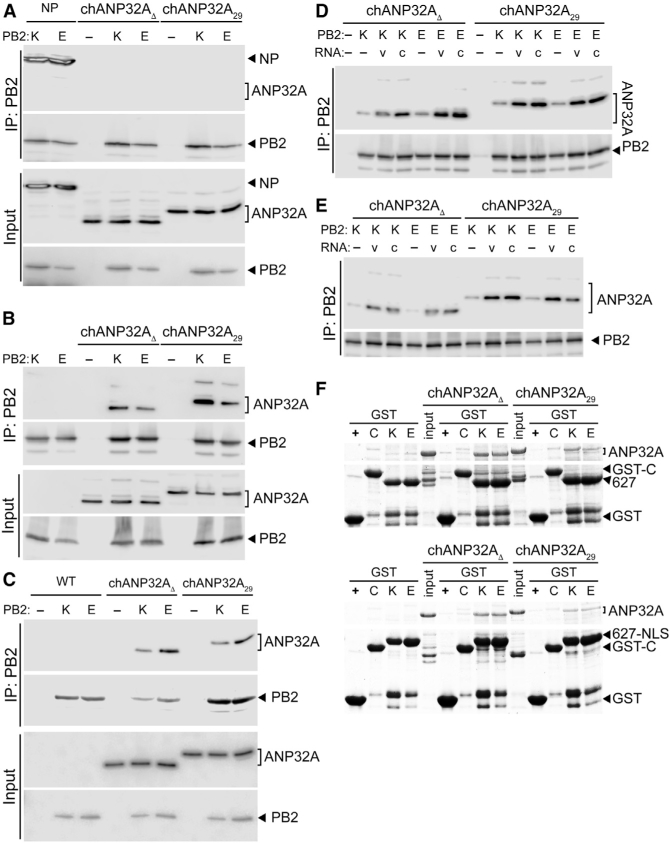
ANP32A Binds Directly to the PB2 627 Domain in a Species-Independent Fashion (A) ANP32A does not stably interact with the PB2 subunit when it is expressed alone. PB2 K627 or E627 was immunoprecipitated (IP) from cells co-expressing ANP32A or the positive control NP. (B) Both chANP32A29 and the humanized chANP32AD interact with the polymerase trimer. Immunoprecipitations were performed from cells expressing ANP32A and the trimeric polymerase containing PB2 K627 or E627. (C) ANP32A interacts with the viral polymerase during infection. WT A549 cells and those stably expressing ANP32A were infected, and PB2 was immunoprecipitated. (D and E) Genomic RNA increases PB2-ANP32A interactions. Immunoprecipitations were performed from cells expressing ANP32A, a vRNA (v) or cRNA (c) genomic segment, and the trimeric polymerase containing PB2 K627 or E627. Binding was measured with catalytically active polymerase (D) or an inactive PB1a mutant polymerase (E). (F) ANP32A binds directly to the PB2 627 domain. In vitro binding was measured between chANP32A variants and GST-tagged PB2 627 domain (top) or PB2 627-NLS (bottom) and visualized by Coomassie staining. GST alone (+) or an irrelevant GST fusion (C) were included as specificity controls.
