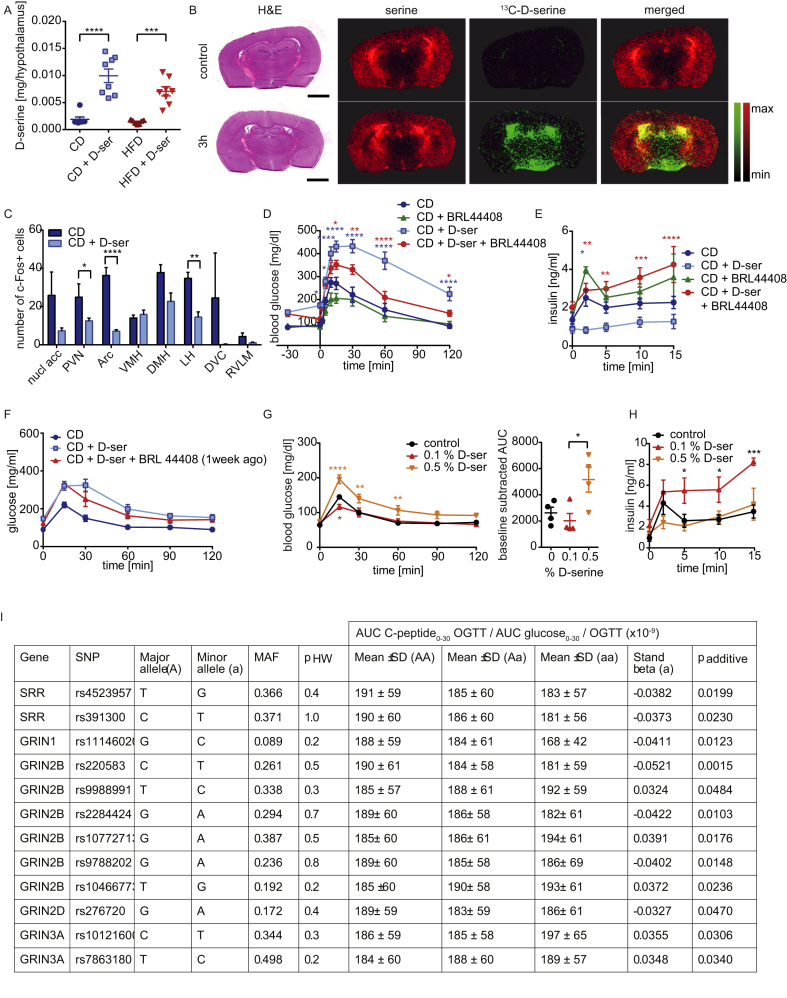
Figure 4.
α2-adrenergic receptor inhibition rescuesd-serine suppressed insulin secretion. (A) Hypothalamic d-serine levels after 8 weeks of d-serine supplementation. (B) 4 week old C57Bl/6 mice were gavaged with 10 mg/kg 13C-labeled d-serine and sacrificed after 3 h. Brains were prepared for MALDI FT-ICR MSI and stained with H&E staining. As negative control, animals were gavaged with water. Size bar represents 2 mm. (C) c-Fos expression in different brain regions after 6 weeks of d-serine supplementation (n = 1–3). Animals were fasted for 6 h prior to the experiment. (D) Glucose and (E) insulin levels during a GSIS after 2 and 4 weeks of d-serine supplementation +/− treatment with the α-adrenergic receptor inhibitor BRL 44408 (5 mg/kg) 30 min prior to glucose administration. (n = 5–10). (F) ipGTT after 3 weeks of d-serine supplementation and one week after a single injection of BRL 44408 (5 mg/kg) (n = 5). (G) ipGTT after 11 days of 0.1 or 0.5% d-serine supplementation (n = 4). (H) GSIS after 4 weeks of d-serine supplementation (n = 4). (I) Hardy–Weinberg equilibrium of genotype distribution analyzed by χ2-test (p HW). Genotype-phenotype association was assessed by multiple linear regression analysis (standard least squares method) in the additive inheritance model (p additive). Insulin secretion data adjusted for gender, age, BMI, and insulin sensitivity are derived from the linear regression models. The effect size of the minor allele is given as standardized beta. AUC – area under the curve; MAF – minor allele frequency; OGTT – oral glucose tolerance test; SD – standard deviation; SNP – single nucleotide polymorphism. Data are shown as mean ± SEM. Statistics were calculated using either ordinary one-way or two-way ANOVA with Tukey's multiple comparison post-hoc test (****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05).