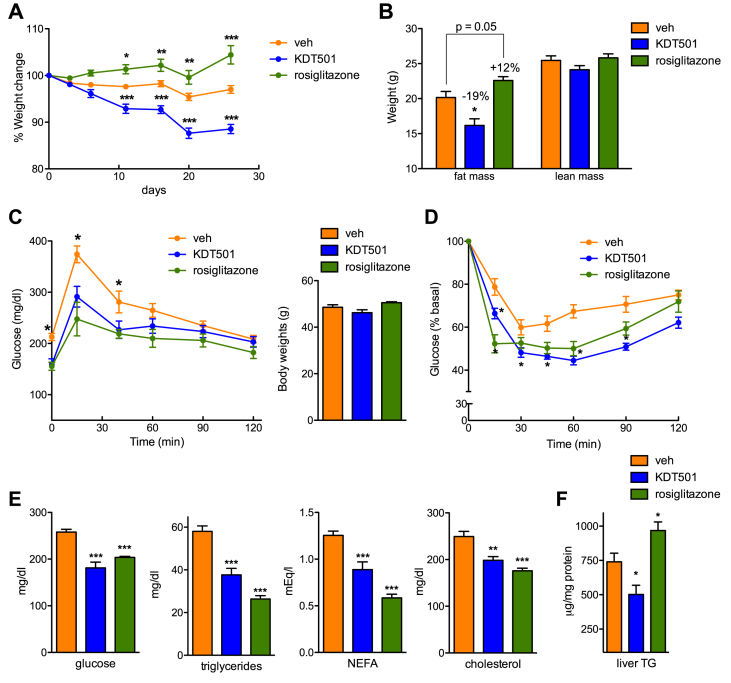
Figure 4.
Chronic KDT501 treatment elicits loss of fat mass and improves plasma lipids. (A and B) Evolution of body weight (A), and final body composition (B) of DIO mice treated with vehicle, 150 mg/kg KDT501, or 10 mg/kg rosiglitazone for 28 days (n ≥ 8). (C) Oral glucose tolerance test in DIO mice treated for 7 days with vehicle, KDT501, or rosiglitazone. Body weights (right panel) were not different at this time (n ≥ 8). (D) Insulin tolerance test after 17 days of treatment (n ≥ 8). (E) Fasted glycemia and plasma lipid profile (n ≥ 7). (F) Hepatic triglyceride (TG) content (n ≥ 7). Data presented as means ± SEM. Statistical significance was calculated using two-way analysis of variance (ANOVA) in A-D, one-way ANOVA in E, and Student's t test in F. *p < 0.05, **p < 0.01, ***p < 0.001 relative to vehicle-treated mice.