Figure 1.
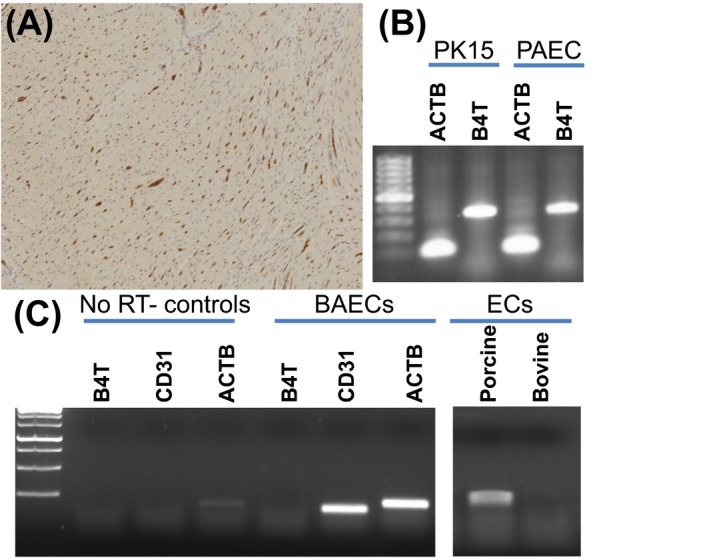
Expression of B4GALNT2 in porcine and bovine cells. A, Dolichos biflorus agglutinin (DBA) lectin staining of Gottingen minipig heart tissue showing capillary endothelial cell staining. B, Reverse transcriptase polymerase chain reaction analysis (RT‐PCR) of B4GALNT2 (B4T) expression in porcine PK15 and porcine aortic ECs (PAEC). RT‐PCR was performed as previously reported 9 using beta‐actin primers (ACTB, forward: CAAGATCATCGCGCCTCCA and reverse: ACTCCTGCTTGCTGATCCACATCT) and B4GALNT2 primers (B4T, forward: TACAGCCCTAGATGTCTGTC and reverse: CTCTCCTCTGAAAGTGTTCGAG). C, RT‐PCR analysis of B4GALNT2 (B4T) total RNA (400 ng/reaction) expression in bovine (BAEC) and porcine endothelial cells (EC). Primers specific for bovine B4GALNT2 (B4T, forward: CTCCAGAGCATTCGTGAGTATT and reverse: TTTGGTGGTGACCTGAGATATG), bovine beta‐actin (ACTB, forward: GTGACATCAAGGAGAAGCTCTG and reverse: AGGAAGGAAGGCTGGAAGA), and CD31 (CD31, forward: GGTCAACGTCACAGAGCTATT and reverse: CACAGTCATGCTTCCCTTCT) were used. Reactions run without a reverse transcriptase step (NO RT controls) show no gene expression. Cultured BAECs show prominent expression of CD31 but do not express B4GALNT2 (1C, left). Additional analysis (1C, right) using RT‐PCR primers conserved in B4GALNT2 in both the porcine and bovine mRNA (forward: ACAAGCTCATGACCATGCTC and reverse: TTTGGTGGTGACCTGAGATATG) detects porcine but not bovine B4GALNT2 expression. RT‐PCR for 1C was performed using one‐step RT‐PCR reaction (USB‐Affymetrix, Santa Clara, CA). Reverse transcription was performed at 42°C for 30 min, followed by 30 cycles of 95°C for 30 s, 58ºC for 30 s, and 72ºC for 50 s, and a final extension for 10 min at 72ºC. Amplification products in 1B and 1C were run in a 1.5% agarose gel
