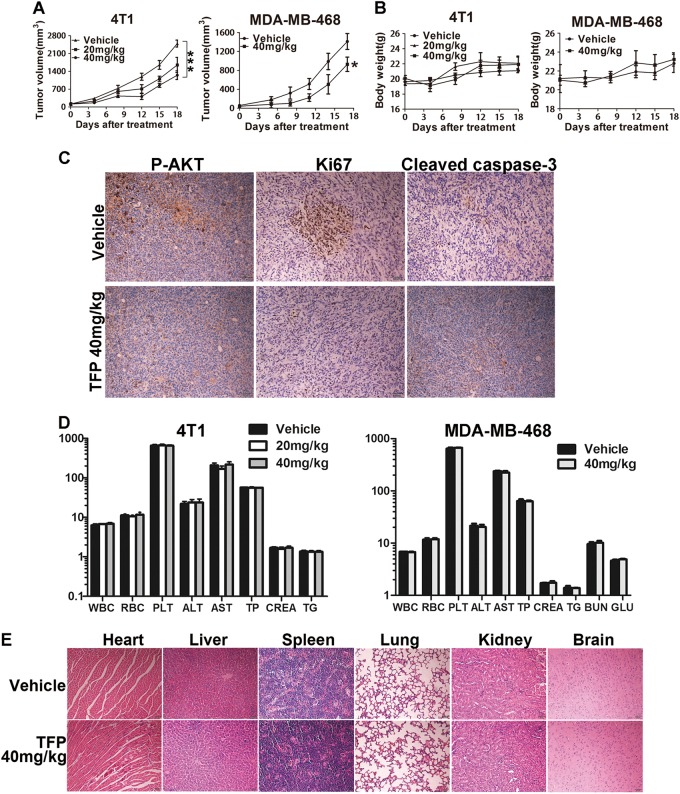
Fig. 5. TFP’s inhibitory effects on the growth of TNBC in subcutaneous tumor model and safety profile.
a Tumor size changes of 4T1-bearing and MDA-MB-468-bearing mice during TFP treatment. Tumor volume and body weight of the mice in each group were measured every 3 days and presented as mean ± SD (n = 7). ANOVA was used for statistical analysis in 4T1 subcutaneous tumor model and t test in MDA-MB-468 subcutaneous tumor model, *P < 0.05; ***P < 0.001. b Body weight changes of mice in each group. There were no significant differences between the groups. c Tumor tissues from the MDA-MB-468 tumor-bearing mice were immunohistochemically analyzed with Ki67, P-AKT, cleaved caspase-3. Scale bars represent 50 μm. d TFP treatment did not cause significant changes in blood routine analysis. Units of the parameters are as follows. WBC, PLT, 109/L; RBC, 1012/L; TP, g/L; ALT, AST, CREA, μM; TG, mM; BUN, GLU, mM. e TFP treatment did not cause obvious pathologic changes in major organs of the mice. Heart, liver, spleen, lung, kidney, and brain were from the mice bearing MDA-MB-468 xenografts tumor. Images shown are representatives from each group. Scale bars represent 50 μm