Fig. 5. Delivery of AAV2 TrkB-2A-mBDNF (2 µl, 1 × 1010 vector particles/eye) to the mouse retina had no adverse effects on retinal health or function.
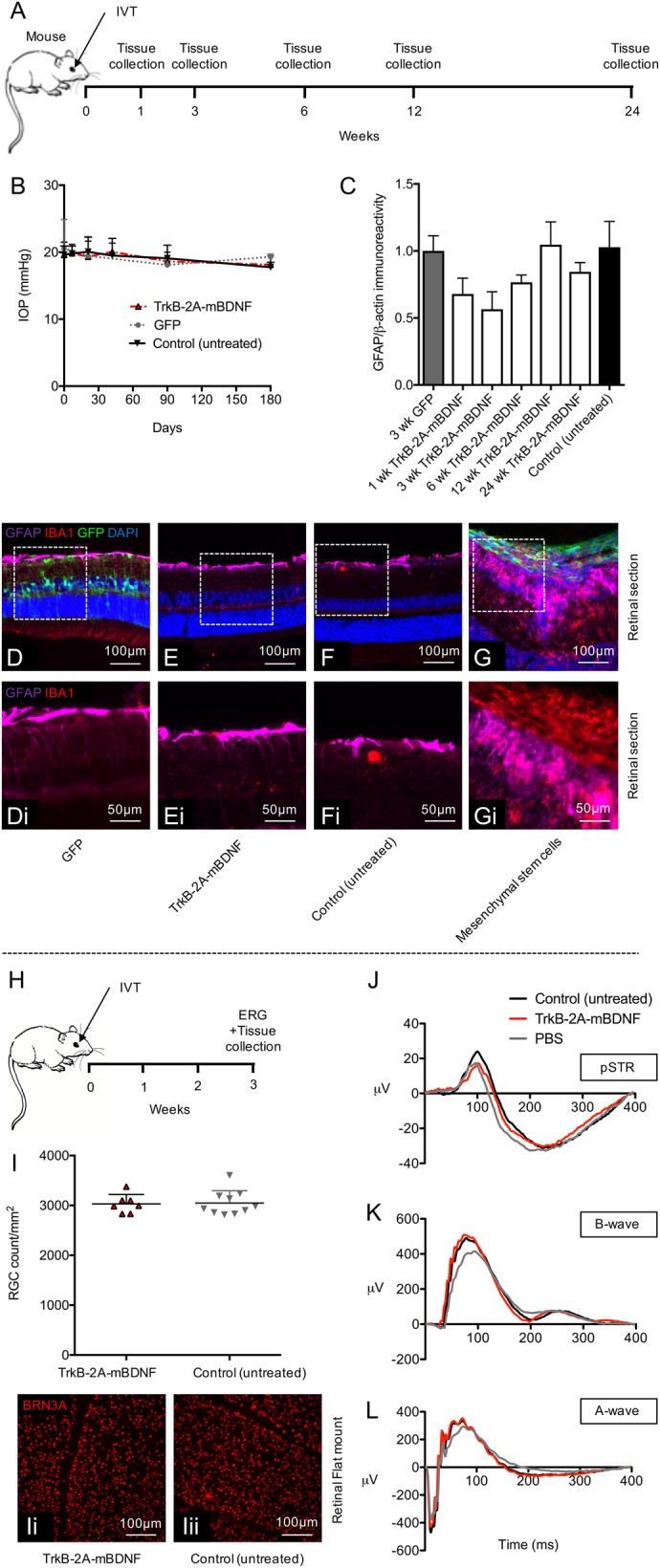
a Schematic of the procedure performed and the time points in which tissues were collected. b Intraocular pressure (IOP) did not change pre- and post-injection with AAV2 GFP or AAV2 TrkB-2A-mBDNF compared to control, non-injected eyes (n = 5/time point). c Gliosis, measured by GFAP expression over time from AAV2 treated retinal lysates compared to control, non-injected eyes (n = 3/time point) also showed no increase in reactivity. d–f Representative images of GFAP immunoreactivity and inflammatory cell marker IBA1 in retinal sections 3 weeks post-treatment. g Gliosis and substantial inflammation was observed 3 weeks after intravitreal injection of 20,000 GFP+ mesenchymal stem cells (2 µl) into the eye, serving as a positive control. i–iii RGC (Brn3A+ cell) counts 3 weeks after intravitreal injection of AAV2 TrkB-2A-mBDNF compared to untreated control eyes showed no detrimental effect on cell number (n = 7–10). j–l Representative pSTRs, characteristic of RGC function, A-wave (bipolar and Müller activity), and mixed A-wave/B-wave (photoreceptor function) profiles between untreated, vector treated, or PBS injected eyes were also not altered when transduced with AAV2 TrkB-2A-mBDNF (n = 7–8) h Schematic of the procedure performed and the time points in which tissues were collected and ERGs recorded
