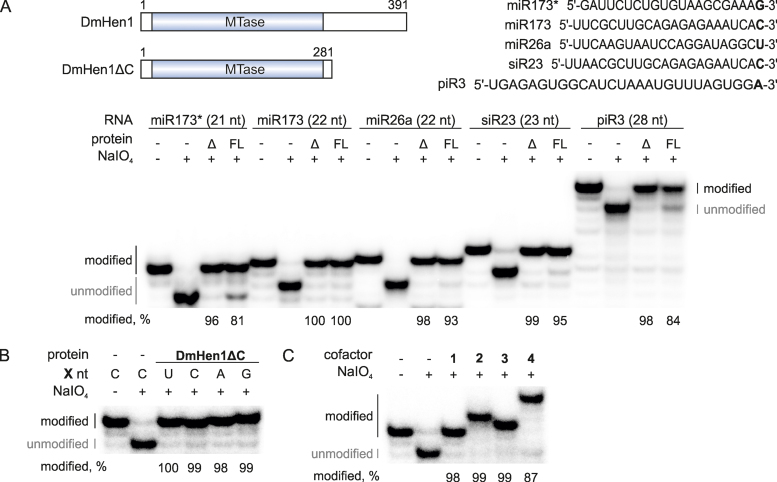
Figure 6.
C-terminal domain of DmHen1 is dispensable for efficient ssRNA modification. (A) DmHen1ΔC methylates RNA substrates of different length and sequence. On the top, schematic representation of DmHen1 and shorter variant lacking C terminal domain DmHen1ΔC, as well as sequences of ssRNA substrates with different 3′-terminal nucleosides (shown in bold). Bottom, methylated RNA separated on denaturing PAA gel with names and lengths of ssRNA indicated above it. Methylation reactions were performed with 2 μM of full length DmHen1 (FL) or truncated DmHen1ΔC (Δ), 0.2 μM of 32P-labelled RNA and 100 μM of AdoMet 1 at 37°C for 30 min. (B) Removal of C terminal domain abolishes DmHen1 bias towards guanosine resulting in full methylation of miR173 with different terminal nucleosides. (C) DmHen1 methyltransferase domain efficiently transfers bulky side chains from synthetic cofactor analogues onto miR173 substrate in the presence of 100 μM of AdoMet 1, Ado-6-amine 2, Ado-6-azide 3 or 50 μM of Ado-13-biotin 4.