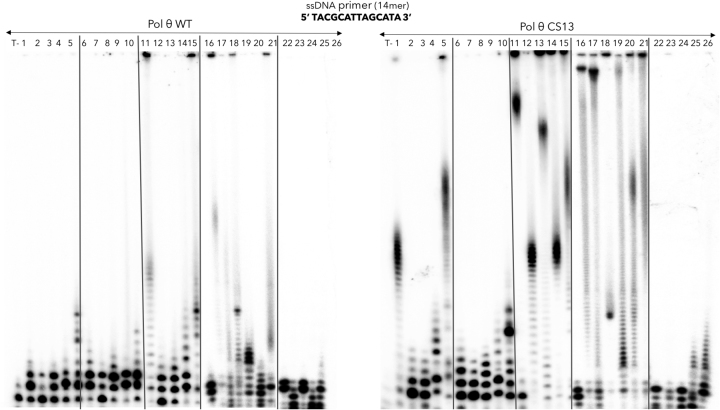
Figure 7.
Incorporation of modified nucleotides by pol θ WT in comparison with the pol θ CS13. The following nucleotide analogs were tested for the elongation of a ssDNA primer by pol θ CS13 compared to pol θ (1) 2′-amino-dATP, (2) 2′-amino-dUTP, (3) 2′-amino-dCTP, (4) 2′-amino-dGTP, (5) mix of 2′-amino-dATP/dUTP/dCTP/dGTP, (6) 2′-O-methyl-dATP, (7) 2′-O-methyl-dUTP, (8) 2′-O-methyl-dCTP, (9) 2′-O-methyl-dGTP, (10) mix of 2′-O-methyl-dATP/dUTP/dCTP/dGTP, (11) 2′-azido-2′-dATP, (12) 2′-azido-2′-dUTP, (13) 2′-azido-2′-dCTP, (14) 2′-azido-2′-dGTP, (15) mix of 2′-azido-2′-dATP/dUTP/dCTP/dGTP, (16) 2′-fluoro-dATP, (17) 2′-fluoro-dUTP, (18) 2′-fluoro-dCTP, (19) 2′-fluoro-dGTP, (20) 2′-fluoro-dTTP, (21) mix of 2′-fluoro-dATP/dUTP/dCTP/dGTP, (22) Ara-ATP (vidarabine triphosphate), (23) Ara-CTP (cytarabine triphosphate), (24) mix of Ara-ATP and Ara-CTP, (25) ϵ-ATP, (26) 2-aminopurine riboside triphosphate. The reactions were performed in the same conditions as the natural NTPs in presence of Mn2+.