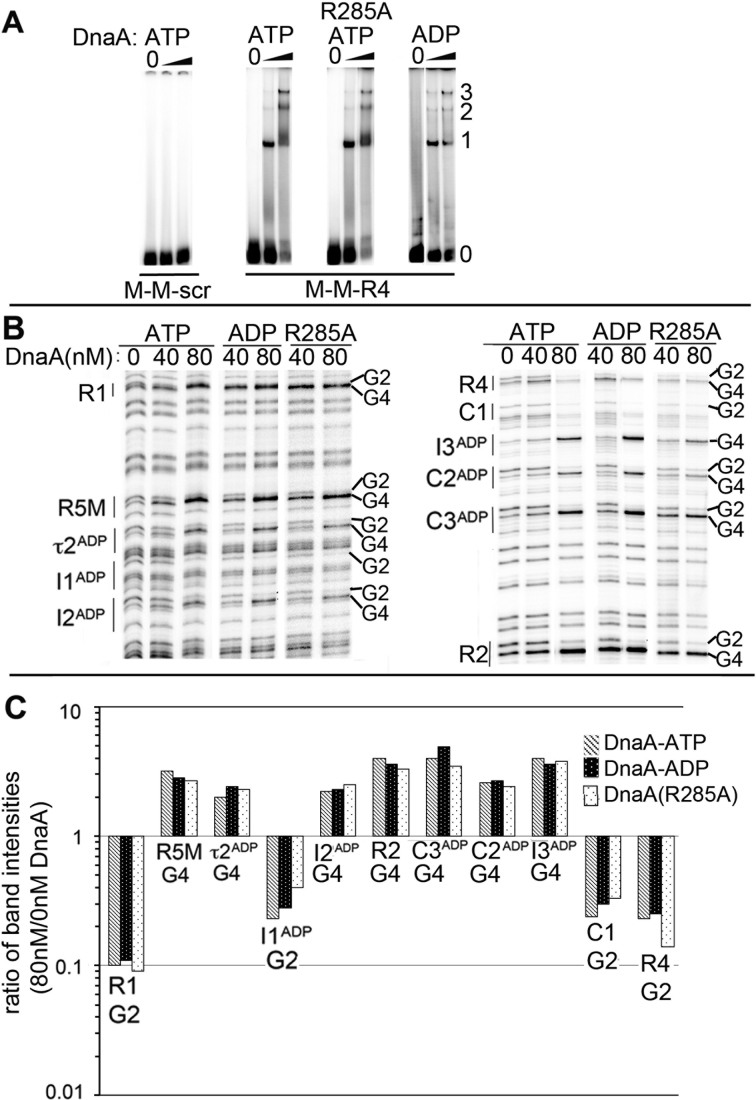
Figure 2.
DnaA-ATP oligomerization is not required for cooperative binding between low affinity sites. (A) EMSA of wild-type DnaA-ATP, DnaA(R285A)-ATP or DnaA-ADP binding to probe carrying the closely spaced sites shown below the panels. DnaA:DNA molar ratios were 0:1, 2.5:1 and 10:1. Positions of unbound probe (0), and probe bound to 1, 2 or 3 molecules of DnaA are marked. (B) DMS footprints of DnaA-ATP, DnaA-ADP and DnaA(R285A)-ATP bound to oriCallADP. Binding sites and diagnostic G2 and G4 residues are marked. (C) Quantitation of G2 or G4 band intensities for each site when bound to 80 nM DnaA-ATP (gray), DnaA-ADP (black) or DnaA(R285A)-ATP (stippled) relative to intensities at 0 nM DnaA.