Figure 7.
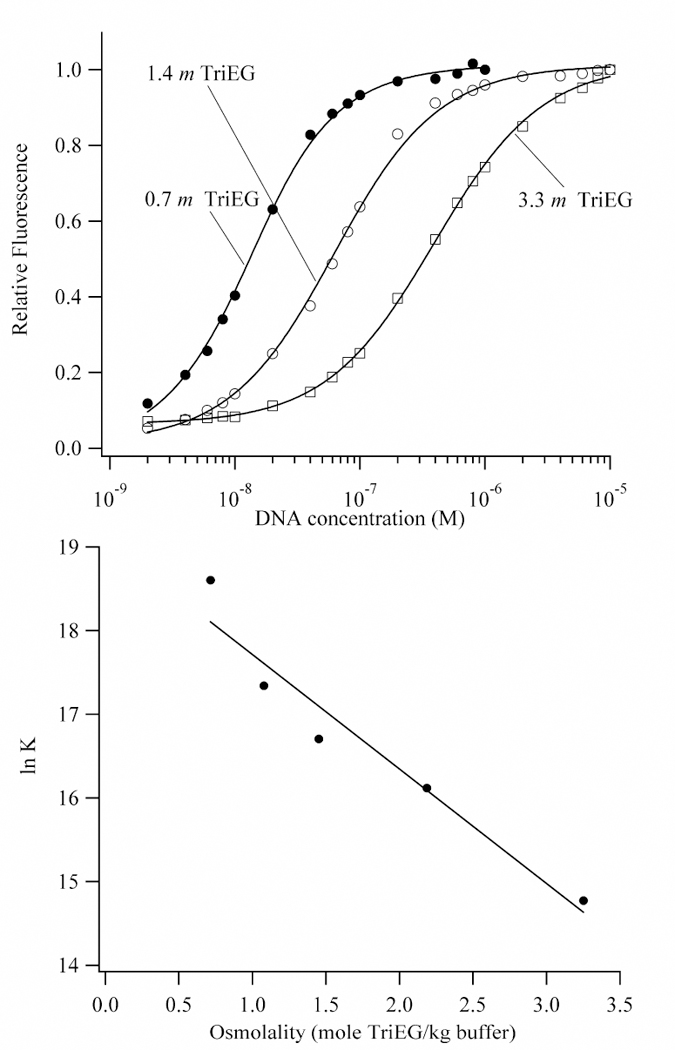
(Top) Binding isotherms for the titration of DNA into Hoechst in buffers with 0.7 m (closed circles), 1.4 m (open circles), and 3.3 m (open squares) triethylene glycol. The affinities of Hoechst for the AATT binding site decreases with increasing concentrations of triethylene glycol, which indicates that water is acquired in the complex. (Bottom) Plot of the natural logarithm of the observed equilibrium constant for the association of Hoechst with DNA as a function of the concentration of triethylene glycol. A linear least-squares fit using Eq. 8 (Kp = 0) gives 78 ± 11 waters that are acquired by the complex. The extrapolation to a 0.12 m for the buffer gives 1.6 (± 0.6) x 108 M−1, which is consistent with the direct measurement of the affinity in buffer alone (2.5 (± 0.4) x 108 M−1) at 25 °C.
