Fig. 1.
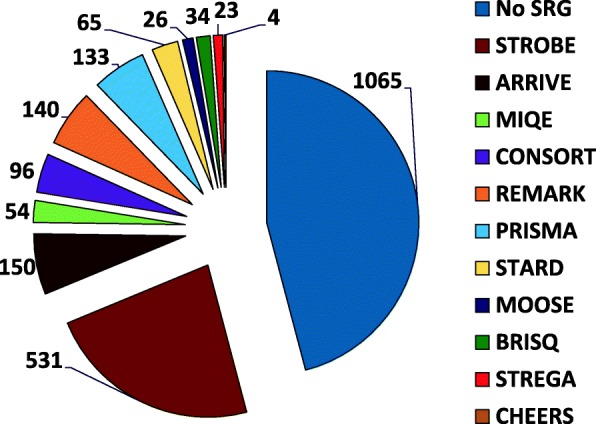
Numbers of submissions for which authors said they used a reporting guideline or did not. Standard reporting guideline (SRG); Strengthening-Reporting of Observational-Studies in Epidemiology (STROBE) [11]; Animal Research: Reporting In Vivo Experiments (ARRIVE) [6]; Minimum Information for Publication of Quantitative Real-Time PCR Experiments (MIQE) [13]; Consolidated Standards of Reporting Trials (CONSORT) [15]; REporting recommendations for tumour MARKer prognostic studies (REMARK) [7]; Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) [10]; studies of diagnostic accuracy (STARD) [8]; Meta-analyses of Observational Studies (MOOSE) [9]; Biospecimen reporting for improved study quality (BRISQ) [14]; STrengthening the REporting of Genetic Association Studies (STREGA) [12], an extension to STROBE; and Consolidated Health Economic Evaluation Reporting Standards (CHEERS) [16]
