Fig. 2.
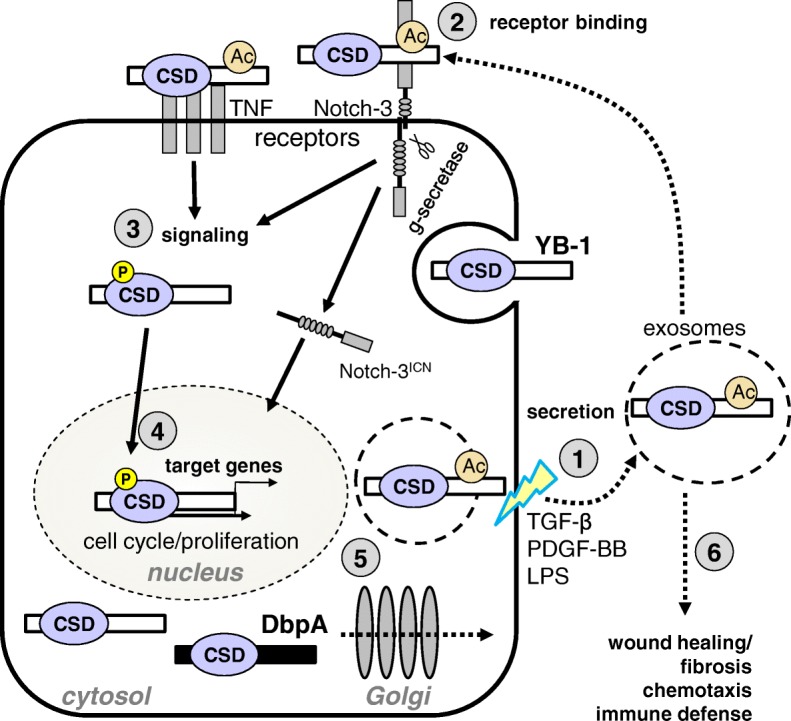
Potential amplification loop for YB-1 in inflammation. (1) Extracellular stimuli (e.g. TGF-β, PDGF-B, LPS) activate cells and induce YB-1 secretion. (2) YB-1 binds to specific membrane associated receptors on the cell surface inducing intracellular signaling cascades that result in kinase activation. YB-1 can also be endocytosed. (3) Activated kinases (e.g. Akt/PKB, ERK, JAK2, RSK) phosphorylate cytoplasmic YB-1 (indicated by the yellow circle), inducing its nuclear translocation. (4) In the nucleus, YB-1 activates the transcription of target genes, as well as induces its own expression and that of DbpA. Cold shock proteins are rapidly induced in response to cell stress, due in part to the existence of preformed complexes of cold shock proteins with their cognate mRNA. (5) Activated cells may also secrete YB-1, which may then act in either an autocrine or paracrine manner. Activated cells may also secrete DbpA via the Golgi. (6) Extracellular YB-1 has mitogenic activity that promotes wound healing/fibrosis. YB-1 also contributes to the recruitment of immune cells to the site of inflammation; directly via its chemoattractant activity or indirectly via the products of its target genes, e.g. CCL5/RANTES. Extracellular activities for DbpA await elucidation. Abbreviations: acetylation (Ac); cold shock domain (CSD); DNA binding protein A (DbpA); lipopolysaccharide (LPS); phosphorylation (P); platelet-derived growth factor B homodimer (PDGF-BB); transforming growth factor beta (TGF-β); tumor necrosis factor (TNF); Y-box binding protein 1 (YB-1)
