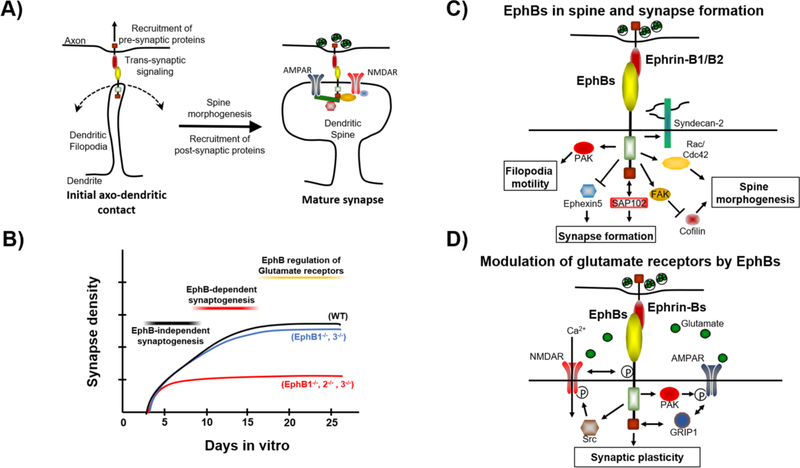
Figure 2.
Post-synaptic EphBs regulate synapse formation and glutamate receptor signaling. (A) Post-synaptic EphBs are required for dendritic filopodia motility, which is necessary for synapse formation. Synapse formation also requires the ability of EphBs to interact with pre-synaptic ephrinBs. (B) EphBs are required for synaptogenesis specifically from approximately DIV7–14 in cortical neurons, after which they remain at synapses and regulate glutamate receptor localization and function. In synaptogenesis EphBs appear to act in a compensatory fashion, with presence of any EphB protein able to rescue the loss of other proteins. This is in contrast to NMDAR localization were loss of EphB2 alone is sufficent to result in decreased synaptic NMDARs both in cortex and hippocampus (2D). (C) EphBs signal through multiple pathways to regulate synapse development. EphB kinase activity results in downstream activation of PAK, which is required for filopodia motility. EphBs also promote synapse formation by promoting degradation of the Rho GEF Ephexin5, and by interacting with the MAGUK scaffolding protein SAP102. EphBs promote spine morphogenesis by interacting with the proteoglycan syndecan-2, by activation of Rac1 GEFs Kalirin and Tiam and the Cdc42 GEF intersectin, and by activation FAK which in turn inhibits the actin severing protein cofilin. Post-synaptic EphBs interact with pre-synaptic ephrin-B1 and ephrin-B2 which mediate pre-synaptic differentiation through interaction with Syntenin-1. (D) At mature synapses, post-synaptic EphBs regulate glutamate receptor signaling. EphBs interact directly with NMDARs and induce Src-mediated phosphorylation of the NMDAR subunit GluN2B at Y1472 which enhances calcium flux through the channel. EphBs also interact indirectly with AMPARs, likely via GRIP1 and induce PAK-mediated phosphorylation of the GluA1 subunit of AMPARs which stabilizes them at the cell surface. EphBs promote NMDAR-dependent LTP, while pre-synaptic ephrin-Bs are required for mossy fiber LTP, which is NMDAR-independent and expressed pre-synaptically.