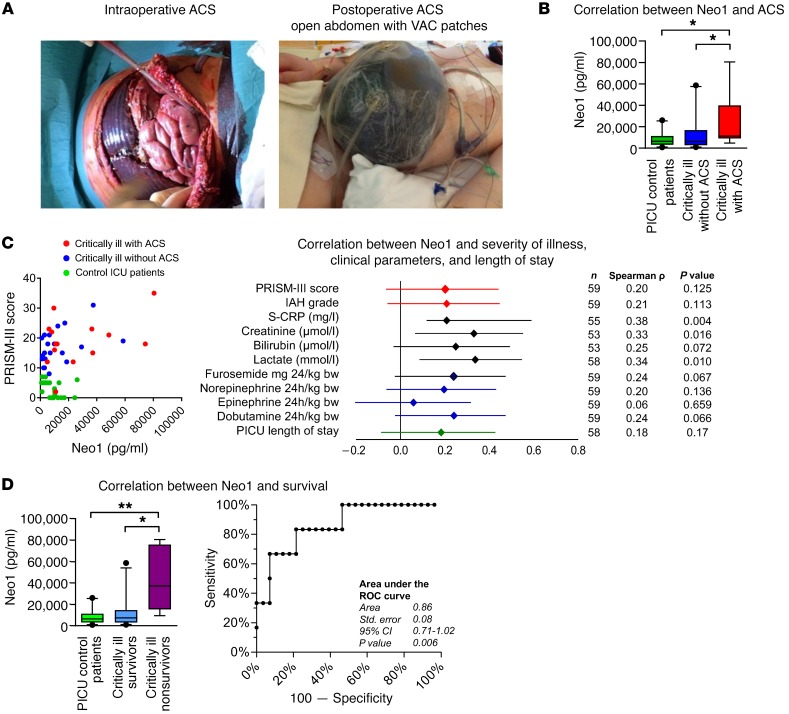
Figure 10. Neo1 in PICU patients with ACS.
Plasma samples from 59 children with and without ACS were collected within 24 hours after admission to the PICU of Hannover Medical School (MHH). (A) Photos displaying the intraoperative situs during open abdomen therapy and the postoperative condition after the establishment of a belly-widening vacuum-assisted closure (VAC) wound dressing in children with fulminant ACS. (B) Value of Neo1 at the admission to the PICU was investigated by comparing the control group (Prism-III score <8), critically ill children without ACS (Prism-III score ≥8), and critically ill children meeting the 2013 WSACS definitions (38) for ACS. (C) Correlation between Neo1 and clinical parameters of all PICU patients enrolled. The Spearman’s rank correlation coefficient Rho and the corresponding 95% CI interval are shown. (D) The predictive value of Neo1 at the admission to the PICU was investigated comparing PICU control patients (PRISM-III score <8), critically ill survivors (PRISM-III score ≥8), and critically ill nonsurvivors. An ROC curve was calculated comparing Neo1 in critically ill survivors with nonsurvivors. Patient characteristics for survivors and nonsurvivors. Results are displayed as median ± 95% CI. Statistical analysis was done by nonparametric Kruskal-Wallis test followed by Dunns post hoc test; correlation was tested using Spearman’s rank correlation test, *P < 0.05, **P < 0.01.