Abstract
The aim of this study was to assess the incidence rate and the risk factors for late complications associated with use of central totally implanted venous access devices (TIVAPs) in patients with cancer, and to devise nursing strategies to minimize late complications.
This retrospective study included 500 patients with TIVAPs from 2012 to 2015. Multivariable logistic regression analysis was performed to assess the effect of sex, age, primary diagnosis, duration of surgery, and the length of hospital stay on the incidence of late complications of TIVAP.
The cumulative maintenance period of TIVAP was 159,605 days. Late complications included catheter-related obstruction (n = 14; 2.8%), infection (n = 3; 0.6%), drug extravasation (n = 1; 0.2%), and catheter exposure (n = 1; 0.2%). Multivariate analyses revealed that age, breast cancer, lung cancer, and gastric cancer were risk factors for the late complications associated with TIVAP.
There was a low incidence of late complications with TIVAP use. Catheter-related obstruction is the most frequent late complication of TIVAP. Risk factors for TIVAP-associated late complications include age and certain cancers, such as breast cancer, lung cancer, and gastric cancer.
Keywords: complications, nursing, risk factors, totally implanted venous access device
1. Introduction
Since its’ first introduction in 1973, totally implantable venous access port (TIVAP; also referred to as device) has been globally used as a safe and stable venous access method for administration of long-term chemotherapy, blood transfusion, and parenteral nutrition. This system consists of a central venous catheter and a subcutaneously-implanted injection port, which provides a simple, safe, and permanent access to the vascular system for intravenous administration of drugs. The port is installed in the skin and is usually connected to the subclavian or the femoral vein (commonly the right) with a catheter.[1] The major advantages of the use of TIVAP include preserving the peripheral blood vessels and allowing the patients to move around, and, therefore, improving the quality of life of the patients. Previous studies have demonstrated that compared with external central venous catheters, the use of TIVAP is associated with a lower risk of infection, is more stable in ambulatory patients, and minimizes the interference in the daily activities of patients.[2–5] However, the use of TIVAP is also associated with several complications, including early complications related to catheter insertion (pneumothorax, arterial perforation, arrhythmias) and late complications (mechanical issues, infection, extravasation, and venous thrombosis).[6,7] The reported incidence of late complications is generally <10%, and most of these are preventable by effective nursing care.[8–10] However, the incidence of late complications in Chinese patients is still unclear. Therefore, this study was designed to determine the incidence of TIVAP-associated late complications and to investigate the risk factors for TIVAP-linked late complications in Chinese patients with cancer, and to suggest appropriate nursing strategies for patient care.
2. Methods
2.1. Patient selection
In this retrospective study, we measured the incidence and analyzed the risk factors for late complications of TIVAP in 500 patients that were treated in the Department of Chemotherapy for cancer at our institution between January 2012 and January 2015. After approval from the Ethics Committee of University Faculty of Medicine, patient-specific data and information were retrieved from the hospital medical records. Written informed consent was obtained from all patients prior to their inclusion.
Patients who qualified all of the following criteria were included in our study: age >18 years; TIVAP at our institution; ability to understand the procedure and provide consent for the study. Patients with serious physical diseases or mental problems, and children with TIVAP were excluded from this study.
All subjects underwent regular preoperative examination, including routine blood examination, coagulation time, and chest fluoroscopy. TIVAP catheters were implanted at the invasive technology department under digital subtraction angiography (DSA) guidance. The TIVAP was implanted by catheterizing either a subclavian vein or the right inguinal vein. The TIVAP, USA BARD (n = 173), and Braun (n = 327), were inserted via right subclavian venous puncture (n = 393), left subclavian venous puncture (n = 104), and right femoral vein puncture (n = 3), and the port was left open. The position of the catheter was confirmed by chest radiography. All patients were successfully catheterized at first attempt. During the treatment period when the TIVAP was used, needles were changed every week; when not in use, the TIVAPs were flushed with diluted heparin solution (100 IU/mL) once a month.
2.2. Follow-Up
After insertion of TIVAP, the patients were followed up on day-14, and 1, 3, and 6 months post-insertion. Data pertaining to age, sex, diagnosis, and surgical history (i.e., vein accessed, whether re-insertion was performed and operation time) were reviewed. Telephonic interviews were conducted to collect information regarding general health, position of port, complications, and nursing strategies used. Complications that occurred after the insertion were recorded as late complications.
2.3. Statistical analysis
Statistical analyses were performed with Stata 13.0 software. Multivariate logistic regression analyses were performed to determine risk factors associated with late complications of TIVAP. Complications were reported in terms of frequency for each type. P < .05 was considered statistically significant.
3. Results
3.1. Demographic characteristics of patients
A total of 500 patients (177 men and 323 women) were included in the study. The mean age (±standard deviation) of patients was 51.8 (±11.43) years. Among these 500 patients, 194 (38.8%) patients had breast cancer, 187 (37.4%) patients had gastrointestinal cancer, 33 (6.6%) patients had lung cancer, 17 (3.4%) patients had hepatic cancer, 12 (2.4%) patients had pancreatic cancer, 12 (2.4%) patients had ovarian cancer, and 45 (9%) patients had other types of cancers. The demographic characteristics of patients are shown in Table 1.
Table 1.
Demographic and basic clinical characteristics of patients.

3.2. Determination of late complications associated with TIVAP
Nineteen out of the 500 patients developed late complications. Of these, 14 (2.8%) patients developed catheter-related obstruction, 3 (0.6%) patients developed infection, 1 (0.2%) patient developed complications associated with drug extravasation, and 1 (0.2%) patient experienced catheter breakage (Table 2). The incidence of the late complications was not significantly different between patients who received BARD and Braun TIVAP (P > .05).
Table 2.
Late complications associated with TIVAP.

3.3. Determination of risk factors for late complications associated with TIVAP
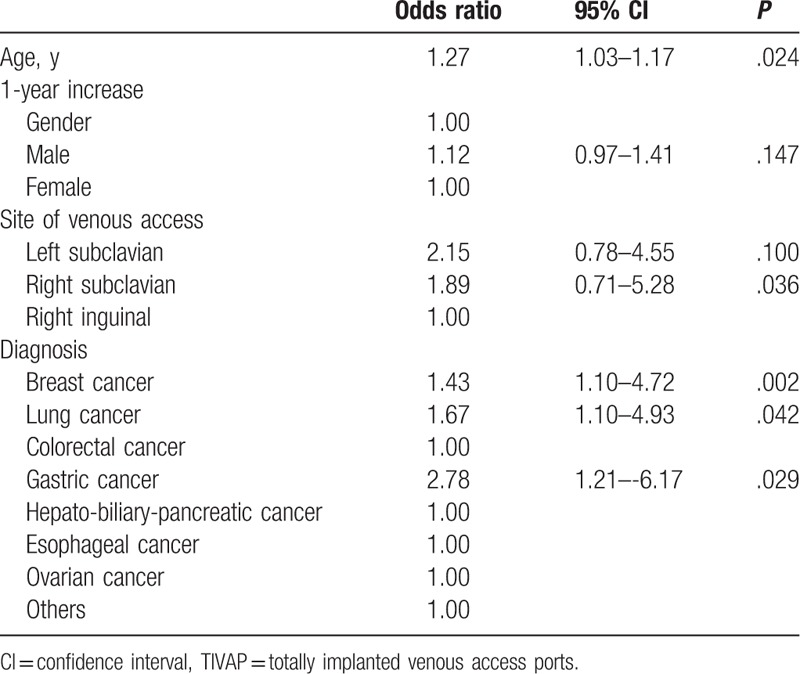
On multivariate analysis, age was found to be a significant risk factor for late complications (odds ratio [OR]: 1.27, 95% CI, 1.03–1.17, P < .05). Neither sex nor site of venous access was associated with late complications (P > .05). In addition, duration of the surgery and the length of hospital stay were also not associated with late complications (P > .05). However, patients with breast cancer (OR: 1.43, 95% CI, 1.10–4.72, P < .01), lung cancer (OR: 1.67, 95% CI, 1.10–4.93, P < .05), and gastric cancer (OR: 2.78, 95% CI, 1.21–6.17, P < .05) showed a significantly higher risk of late complications (Table 3).
Table 3.
Risk factors for late complications associated with TIVAP.
4. Discussion
Use of TIVAP is a standard practice in patients on long-term chemotherapy, blood transfusion, and parenteral nutrition across the world. However, no guidelines regarding the application of TIVAP are available in China to date. As described in previous studies, use of TIVAP involves several side effects and complications, which could add to the physical and psychological burden on the patients.[11] Associated complications may even cause failure of the ports.[12,13] In this study, we assessed data pertaining to 500 patients with TIVAP at our hospital to profile the risk factors, which could help inform practices related to TIVAP use.
The overall incidence of late complications in this relatively large cohort was 3.8%, which was lower than that reported from previous studies.[14–16] We believe that this is primarily attributable to the careful preoperative preparation and intraoperative processing. In addition, the complications were mainly reported by the patients during the follow up; thus, some of the relatively minor complications may not have been reported by the patients. Mechanical complications were the most common late complications, followed by infection, which is similar to that reported earlier.[17] Children showed a greater predilection for severe late complications, which may explain the higher incidence rate reported in children in previous studies.[15,18] Complications related to catheter obstruction were the most common among patients with long-term in-dwelling TIVAP. These complications included slow catheter infusion and failure to transfuse or draw back blood. There were 2 types of catheter-related obstructions, namely thrombotic and non-thrombotic obstruction. Non-thrombotic obstruction refers to obstruction caused by mechanical factors, including physical distortion or folding of the catheter and/or the infusion apparatus, or by deposition of drug. In our study population, 13 patients developed thrombotic obstruction, while 1 patient developed non-thrombotic obstruction. Thrombotic obstructions were dealt with 1 mL heparin diluents, which successfully resolved the obstruction in all 13 patients. In patients with mechanical thrombotic obstruction, the TIVAP was manipulated under DSA guidance, which was dragged to the right subclavian vein.[7]
Infectious complications included both systemic and local infections. Local infection refers to skin/soft-tissue infection at the site of venous access and tunnel infection. Systemic catheter infection refers to catheter-related bloodstream infection (CR-BSIs), and was defined as the presence of general systemic symptoms and the absence of any other obvious source of infection. The incidence of infection in this study (0.6%) was much lower than that reported earlier.[19] This is likely attributable to the following factors: the operation procedures in this study were performed carefully, and all patients received appropriate postoperative care; the overall incidence of complications was very low. Therefore, there were fewer conditions to trigger infection; and some “minor” infections might not have been reported by the patients. In addition, similar pathogens were isolated on semi-quantitative and quantitative culture of peripheral blood samples. There are 3 main modes of infection associated with use of catheter: infections caused by bacteria present on the surface of the skin during the process of TIVAP; catheter-related bloodstream infection leading to CR-BSI; microbial contamination and cavity infection caused by bacteria. There was one case of local infection which finally progressed to CR-BSIs, which manifested as unexplained fever and positive blood culture The patient recovered with antibiotic treatment.[20]
Drug extravasation refers to the leakage of liquid drug or penetration into subcutaneous due to the falling-off of non-damage needle, shortage of needles, dislodgement of the catheter, and tube rupture. We had 1 case with drug extravasation in this study. Symptoms of drug extravasation included local subcutaneous swelling, burning sensation and pain; these were resolved after local hydropathic compresses and treatment of blockage. There was one case of catheter exposure and was taken out by surgical intervention.[21]
In the present study, sex of the patient and the site of venous access were not risk factors for late complications after TIVAP application. However, age and certain underlying diseases were associated with a higher risk of late complications. In this study cohort, every 1 year increase in age was associated with a 0.27-fold higher risk of late complications associated with use of TIVAP, which is consistent with that reported elsewhere.[22]
The association of age with late complications is likely attributable to age-related vascular changes and to the relatively poor general health situation.[23,24] Patients with breast cancer, lung cancer, and gastric cancer had a higher rate of complication. This is likely attributable to the higher rate of indwelling TIVAP in patients with these cancer types as compared with that in patients with other cancers in our hospital. We can increase the sample size for further in-depth consideration.
Our study had several limitations. First, this study was a single-center study and the method used for obtaining data was related to late complications, which may have introduced sampling bias. Second, our study was a retrospective observational study, which is inherently liable to bias. Future multi-centered studies with large cohorts are needed to further corroborate our findings.
Catheter obstruction was the most frequent type of late complication in this study. Given that most catheter obstructions could be spotted by careful postoperative care, we suggest that the nurses should pay more attention to prevent catheter obstruction. For example, x-ray may be used to check for any change in position of the port. Also, in order to prevent blood clots and catheter obstruction, nurses should inject not <10 mL normal saline (NS) to seal the tube by positive pressure. Moreover, nurses should pay more attention to drug compatibility to minimize the possibility of interaction between different drugs leading to catheter blockage. In the event of incomplete obstruction, nurses should draw the fluid as soon as possible and gently inject 1 mL urokinase. If the obstruction accident cannot be solved, nurses should arrange the patients to take out the catheter as soon as possible.[13]
Adherence to aseptic principles during implantation of the catheter or insertion of the non-damage indwelling needle, or during change of dressing is essential. If the patients need long-term transfusion, one suit of non-damage needles could be used for 7 days in a row.[25] Dressing should be of permeable materials and it should be changed as soon as possible once the dressing falls off. Professional non-damage needles should be used according to the venous access port. Local skin care of the patients with TIVAP is another important aspect of care. The infusion ports are inserted into the subcutaneous tissue, which will impair the local skin elasticity and liable to impair blood circulation in the subcutaneous tissue. Repeated punctures and the side effects of the chemotherapy drugs also lead to impaired immunity. Therefore, nurses should educate and instruct the patients to take care of the skin around the infusion ports. Wound management and general anti-infection treatment are required for any infection. The infusion ports should not be used prior to complete control of the infection. There are 2 types of treatments of CR-BSIs: sealing with antibiotics and catheter removal. Though it is a useful method to remove the tube, yet the tube may actually be uninfected. Nurses could choose to seal the tube with NS to both avoid chances of infection during tubes removal and to allow for healing of the central venous catheter infection. Nurses should master the diagnosis of catheter-related infections and the indications and contraindications to use of antibiotics sealing tubes.[26]
Patients should be educated to avoid any movement that increases skin tension near the infusion ports, such as strenuous outreach activities and chest expanding exercises to prevent drug extravasation due to falling off of the needle. In the event of any drug extravasation, nurses should terminate transfusion as soon as possible and draw the liquid residue with an empty syringe. Upper limbs should be elevated and 50% magnesium sulfate should be applied to serve as a humid heat compress. Local seal should be applied by the nurses according to the drug extravasation situation and alongside monitor the skin situation. Once the tube falls off, prompt intervention with doctor's help is required. In the event of a catheter rupture, nurses should report to the doctors and comfort the patients and the families.[27] In order to minimize the chances of drug extravasation, TIVAP should be applied by qualified nurses with certain certification.[28] High-pressure injection of contrast agent should be strictly prohibited. Patients who are suffering from pinch-off syndrome should be educated by the nurses to re-check the chest x-ray regularly. Health education for TIVAP management should be conducted by nurses. As the injection position is under subclavian near the epidermis, it is easy to rub the subcutaneous tissue while pulling, which renders the skin thinner and may eventually lead to skin breakage. Nurses should educate the patients with implanted TIVAP to avoid strenuous exercise on the infusion port side and to not to scrub the skin near the port while taking showers to take care of the skins.[29,30]
5. Conclusion
In the present study, we report that catheter-related obstruction is the most frequent late complication associated with TIVAP. Risk factors for TIVAP-linked late complications include age and certain cancers, such as breast cancer, lung cancer, and gastric cancer.
Author contributions
Conceptualization: Xin-Yan Yu, Jia-Lan Xu, Dan Li, Zi-Fang Jiang.
Data curation: Xin-Yan Yu, Jia-Lan Xu, Dan Li, Zi-Fang Jiang.
Formal analysis: Xin-Yan Yu, Jia-Lan Xu, Dan Li, Zi-Fang Jiang.
Funding acquisition: Xin-Yan Yu, Jia-Lan Xu, Dan Li, Zi-Fang Jiang.
Investigation: Xin-Yan Yu, Jia-Lan Xu, Dan Li, Zi-Fang Jiang.
Methodology: Xin-Yan Yu, Jia-Lan Xu, Dan Li, Zi-Fang Jiang.
Project administration: Xin-Yan Yu, Jia-Lan Xu, Dan Li, Zi-Fang Jiang.
Resources: Xin-Yan Yu, Jia-Lan Xu, Dan Li, Zi-Fang Jiang.
Software: Xin-Yan Yu, Jia-Lan Xu, Dan Li, Zi-Fang Jiang.
Supervision: Xin-Yan Yu, Jia-Lan Xu, Dan Li, Zi-Fang Jiang.
Validation: Xin-Yan Yu, Jia-Lan Xu, Dan Li, Zi-Fang Jiang.
Visualization: Xin-Yan Yu, Jia-Lan Xu, Dan Li, Zi-Fang Jiang.
Writing – original draft: Xin-Yan Yu, Jia-Lan Xu, Dan Li, Zi-Fang Jiang.
Writing – review & editing: Xin-Yan Yu, Jia-Lan Xu, Dan Li, Zi-Fang Jiang.
Footnotes
Abbreviations: CR-BSIs = catheter-related bloodstream infection, DSA = digital subtraction angiography, TIVAP = totally implanted venous access port.
This work was supported by the Medical and Health Science and Technology Project of Zhejiang Province: class A, project number: 2015118745, implantable venous infusion port of the maintenance interval extension of the treatment period.
The authors declare that they have no conflict of interest.
References
- [1].Goltz JP, Petritsch B, Kirchner J, et al. Percutaneous image-guided implantation of totally implantable venous access ports in the forearm or the chest? A patients’ point of view. Support Care Cancer 2013;21:505–10. [DOI] [PubMed] [Google Scholar]
- [2].Di Carlo I, Cordio S, La Greca G, et al. Totally implantable venous access devices implanted surgically: a retrospective study on early and late complications. Arch Surg 2001;136:1050–3. [DOI] [PubMed] [Google Scholar]
- [3].Vescia S, Baumgartner AK, Jacobs VR, et al. Management of venous port systems in oncology: a review of current evidence. Ann Oncol 2008;19:9–15. [DOI] [PubMed] [Google Scholar]
- [4].Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc 2006;81:1159–71. [DOI] [PubMed] [Google Scholar]
- [5].O’Grady NP, Alexander M, Dellinger EP, et al. Guidelines for the prevention of intravascular catheter-related infections. Infect Control Hosp Epidemiol 2002;23:759–69. [DOI] [PubMed] [Google Scholar]
- [6].Kurul S, Saip P, Aydin T. Totally implantable venous-access ports: local problems and extravasation injury. Lancet Oncol 2002;3:684–92. [DOI] [PubMed] [Google Scholar]
- [7].Gallieni M, Pittiruti M, Biffi R. Vascular access in oncology patients. CA Cancer J Clin 2008;58:323–46. [DOI] [PubMed] [Google Scholar]
- [8].Yildizeli B, Lacin T, Batirel HF, et al. Complications and management of long-term central venous access catheters and ports. J Vasc Access 2004;5:174–8. [DOI] [PubMed] [Google Scholar]
- [9].Arch P. Port navigation: let the journey begin. Clin J Oncol Nurs 2007;11:485–8. [DOI] [PubMed] [Google Scholar]
- [10].Dougherty L. Maintaining vascular access devices: the nurse's role. Support Care Cancer 1998;6:23–30. [DOI] [PubMed] [Google Scholar]
- [11].Lebeaux D, Larroque B, Gellen-Dautremer J, et al. Clinical outcome after a totally implantable venous access port-related infection in cancer patients: a prospective study and review of the literature. Medicine (Baltimore) 2012;91:309–18. [DOI] [PubMed] [Google Scholar]
- [12].Sarper N, Zengin E, Corapcioglu F, et al. Totally implantable central venous access devices in children with hemato-oncologic malignancies: evaluation of complications and comparison of incidence of febrile episodes with similar patients without central venous access devices. Pediatr Hematol Oncol 2006;23:459–70. [DOI] [PubMed] [Google Scholar]
- [13].Zaghal A, Khalife M, Mukherji D, et al. Update on totally implantable venous access devices. Surg Oncol 2012;21:207–15. [DOI] [PubMed] [Google Scholar]
- [14].Dal Molin A, Rasero L, Guerretta L, et al. The late complications of totally implantable central venous access ports: the results from an Italian multicenter prospective observation study. Eur J Oncol Nurs 2011;15:377–81. [DOI] [PubMed] [Google Scholar]
- [15].Dillon PA, Foglia RP. Complications associated with an implantable vascular access device. J Pediatr Surg 2006;41:1582–7. [DOI] [PubMed] [Google Scholar]
- [16].Bassi KK, Giri AK, Pattanayak M, et al. Totally implantable venous access ports: retrospective review of long-term complications in 81 patients. Indian J Cancer 2012;49:114–8. [DOI] [PubMed] [Google Scholar]
- [17].Nam SH, Kim DY, Kim SC, et al. Complications and risk factors of infection in pediatric hemato-oncology patients with totally implantable access ports (TIAPs). Pediatr Blood Cancer 2010;54:546–51. [DOI] [PubMed] [Google Scholar]
- [18].Rauthe G, Altmann C. Complications in connection with venous port systems: prevention and therapy. Eur J Surg Oncol 1998;24:192–9. [DOI] [PubMed] [Google Scholar]
- [19].Lebeaux D, Fernandez-Hidalgo N, Chauhan A, et al. Management of infections related to totally implantable venous-access ports: challenges and perspectives. Lancet Infect Dis 2014;14:146–59. [DOI] [PubMed] [Google Scholar]
- [20].El Hammoumi M, El Ouazni M, Arsalane A, et al. Incidents and complications of permanent venous central access systems: a series of 1,460 cases. Korean J Thorac Cardiovasc Surg 2014;47:117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Weingart SN, Hsieh C, Lane S, et al. Standardizing central venous catheter care by using observations from patients with cancer. Clin J Oncol Nurs 2014;18:321–6. [DOI] [PubMed] [Google Scholar]
- [22].Hsieh CC, Weng HH, Huang WS, et al. Analysis of risk factors for central venous port failure in cancer patients. World J Gastroenterol 2009;15:4709–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lipitz-Snyderman A, Sepkowitz KA, Elkin EB, et al. Long-term central venous catheter use and risk of infection in older adults with cancer. J Clin Oncol 2014;32:2351–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Singh KR, Agarwal G, Nanda G, et al. Morbidity of chemotherapy administration and satisfaction in breast cancer patients: a comparative study of totally implantable venous access device (TIVAD) versus peripheral venous access usage. World J Surg 2014;38:1084–92. [DOI] [PubMed] [Google Scholar]
- [25].Biffi R, Toro A, Pozzi S, et al. Totally implantable vascular access devices 30 years after the first procedure. What has changed and what is still unsolved? Support Care Cancer 2014;22:1705–14. [DOI] [PubMed] [Google Scholar]
- [26].Koutsopoulou S, Papathanassoglou ED, Katapodi MC, et al. A critical review of the evidence for nurses as information providers to cancer patients. J Clin Nurs 2010;19:749–65. [DOI] [PubMed] [Google Scholar]
- [27].Biffi R, Pozzi S, Agazzi A, et al. Use of totally implantable central venous access ports for high-dose chemotherapy and peripheral blood stem cell transplantation: results of a monocentre series of 376 patients. Ann Oncol 2004;15:296–300. [DOI] [PubMed] [Google Scholar]
- [28].Cardoso AF, Moreli L, Braga FT, et al. Effect of a video on developing skills in undergraduate nursing students for the management of totally implantable central venous access ports. Nurse Educ Today 2012;32:709–13. [DOI] [PubMed] [Google Scholar]
- [29].Piredda M, Biagioli V, Giannarelli D, et al. Improving cancer patients’ knowledge about totally implantable access port: a randomized controlled trial. Support Care Cancer 2016;24:833–41. [DOI] [PubMed] [Google Scholar]
- [30].Kullberg A, Sharp L, Johansson H, et al. Information exchange in oncological inpatient care–patient satisfaction, participation, and safety. Eur J Oncol Nurs 2015;19:142–7. [DOI] [PubMed] [Google Scholar]


