Abstract
Background
Upright T wave in lead aVR (TaVR) has recently been reported to be associated with cardiovascular death and mortality in general population and in patients with prior cardiovascular disease (CVD). However, the evidence for the predictive ability of TaVR in patients with ischemic stroke (IS) is lacking.
Methods
A total of 625 consecutive patients with IS (mean age: 66 ± 12 years; 379 male) were enrolled in this study between January 2013 and December 2014. Patients were divided into upright TaVR (≥0 mV; n = 201) and negative TaVR (<0 mV; n = 424) groups. All patients were evaluated with respect to clinical features and in-hospital clinical results.
Results
Overall, the prevalence of upright TaVR was 32.2% at baseline. Patients with an upright TaVR were older, had a higher percentage of CVD and hypertension, higher level of MB isoenzyme of creatine kinase (CKMB), faster heart rate, higher rate of QT prolongation > 450 ms, higher rate of negative T in lead II, higher rate of negative T in lead V6, higher rate of ST depression, and longer QTc duration. During the mean follow-up period of 20.0 ± 5.8 months, 29 (4.6%) patients experienced all-cause death and 12 (1.9%) patients experienced cardiovascular death, the primary end point. Concomitantly, 94 (15%) patients experienced recurrence of IS, the secondary end point. After adjusting for clinical covariates, upright TaVR was independently associated with all-cause death [hazard ratio (HR): 2.88, 95% confidence intervals (CI): 1.07–7.73], cardiovascular death (HR: 3.04, 95% CI: 1.07–8.64), and IS recurrence (HR: 1.86, 95% CI: 1.08–3.20).
Conclusions
Upright TaVR in patients with IS is associated with increased mortality and recurrence of IS.
Keywords: T wave in lead aVR, Ischemic stroke, Prognosis
Introduction
Electrocardiography (ECG) is a routine investigation in patients with stroke and provides essential information about underlying ischemic stroke (IS) etiology. Little evidence, however, exists concerning the prevalence of ECG changes and their prognostic impact for patients with IS.1 According to previous studies, the most common ECG abnormalities after IS are QT-prolongation, cardiac ischemic changes (T-wave inversion and ST-segment depression), and non-hyperkalemia-dependent U-waves.2, 3 However, the prognostic role of T-wave changes in lead aVR has long been underestimated. Lead aVR, an augmented and unipolar limb lead, was constructed to obtain specific information from the right upper part of the heart, including the outflow tract of the right ventricle and the basal portion of the interventricular septum. Several recent studies have validated the role of upright T-wave amplitude in lead aVR (TaVR) as a powerful, independent prognostic predictor of cardiovascular mortality in the general population,4, 5, 6 as well as in some clinical settings.7, 8, 9, 10 However, neither the prevalence, nor the predictive value of upright TaVR, has been evaluated in patients with IS.
We designed this study to evaluate whether upright TaVR is a useful predictor of short-term prognosis in patients with IS.
Methods
Subjects
Eligible patients diagnosed with IS at Renmin Hospital of Wuhan University between January 2013 and December 2014 were enrolled in this study. The exclusion criteria were as follows: (1) non-ischemic stroke (i.e., hemorrhagic stroke); (2) ECG not available; (3) atrial fibrillation; (4) right or left bundle branch block; (5) sick sinus syndrome; (6) presence of cardiac pacing or a defibrillator. The present study was conducted in accordance with the Declaration of Helsinki and was approved by the ethical committee of our hospital.
Electrocardiogram
Twelve-lead ECG (paper speed 25 mm/s; amplitude 1.0 mV/10 mm) was recorded in all patients soon after their admission to the hospital. Upright TaVR was defined as a wave with a positive deflection ≥0 mV, and negative TaVR was defined as TaVR <0 mV. Heart rate, QRS complex duration, and QT interval were recorded automatically by the ECG machine. The corrected QT (QTc) was adjusted for the RR interval, using the Bazett formula (). An abnormal Q wave was defined as a wave of ≥20 ms in duration or a QS complex in leads V2—V3 and a wave ≥ 30 ms in duration and ≥1 mm in depth, or a QS complex in other leads. Negative T wave in lead II was defined as a wave <0 mV in lead II. Negative T wave in lead V6 was defined as a wave <0 mV in lead V6. ST depression was defined as a drop of ST segment >0.5 mm in lead V5 from the isoelectric line.
Blood test
Blood test was performed before discharge from the hospital. The blood test included serum potassium (K), sodium (Na), calcium (Ca), B-type natriuretic peptide (BNP), MB isoenzyme of creatine kinase (CKMB), troponin I (cTnI), homocysteine (HCY), uric acid (UA), total glyceride (TG), total cholesterol (TC), and low-density lipoprotein cholesterol (LDL) levels.
Definitions
Hypertension was defined as a systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥90 mmHg, or treatment with antihypertensive drugs. Diabetes mellitus was considered to be present in patients with diabetes controlled by diet, oral hypoglycemic agents, or insulin, as well as in cases discharged from the hospital with a diagnosis of diabetes mellitus and/or prescription of hypoglycemic agents. Hyperlipidemia was defined as the use of lipid-lowering agents, a total serum cholesterol level >240 mg/dl, or a serum triglyceride level >200 mg/dl. In the present study, cardiovascular disease (CVD) included ischemic heart disease that had been diagnosed as acute myocardial infarction (MI), coronary stenosis detected by coronary angiography and treated by percutaneous coronary revascularization, and/or coronary artery bypass grafting, IS, hemorrhagic stroke, peripheral artery disease, and history of macrovascular surgery.
Follow-up and end points
The subjects were followed-up until March 31, 2017. Research coordinators and physicians recorded baseline data of all patients at the time of enrollment, including patient demographics, past medical conditions, and current medication. During the follow-up period, patients or their families were periodically sent a questionnaire and interviewed by telephone. The primary end point of the study included all-cause death and cardiovascular death; cardiovascular death included death due to heart failure, acute MI, aortic dissection, or systemic embolism. The causes of death (including cardiovascular death) were determined from medical records or by direct communication with patients' general practitioners or families.
The secondary end point was unplanned rehospitalization for recurrence of IS. The secondary end point was determined from medical records.
Statistical analysis
Data were expressed as mean ± standard deviation or percentages. Comparisons of continuous variables were performed using the unpaired t-test. Comparisons of categorical variables were performed using the chi-squared test. Associations of TaVR with the end points were analyzed using the univariate and multivariate Cox proportional hazards regression models and presented as a hazard ratio (HR) and 95% confidence interval (CI). The following explanatory variables were used in the univariate analyses: age, male sex, CVD, hypertension, heart rate, QTc, CKMB, HCY, statin use, QT prolongation >450 ms, negative T in lead II, negative T in lead V6, ST depression, and upright TaVR. Explanatory variables with a P value < 0.05 in the univariate analysis were entered into multivariate analysis. P values < 0.05 were considered statistically significant. All statistical analyses were performed using SPSS for Windows version 20 (IBM Corp., Armonk, NY, USA).
Results
Patients' characteristics at baseline
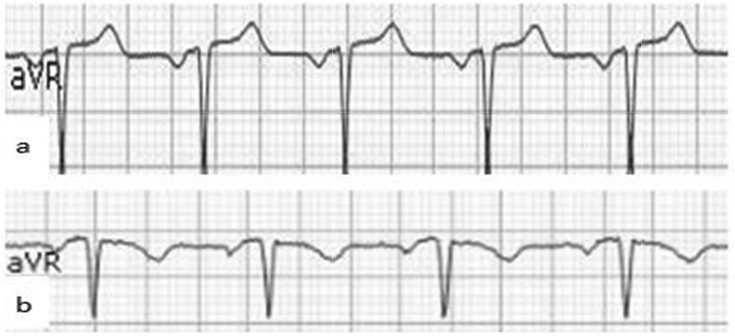
A total of 709 consecutive patients were assessed for enrollment eligibility. We excluded 84 patients due to predetermined criteria, resulting in a total of 625 patients (379 male and 246 female, mean age 66 ± 12 years) who were enrolled in this study. Fig. 1 shows the representative ECG of upright TaVR (Fig. 1a) and negative TaVR (Fig. 1b). Among the 625 patients, 201 (32%) patients had upright TaVR and 424 (68%) had negative TaVR. Table 1 lists the baseline characteristics of patients. Significant differences were noted between the two groups in terms of age, percentage of CVD and hypertension, heart rate, QT prolongation >450 ms, negative T in lead II, negative T in lead V6, ST depression, QTc duration, and serum CKMB levels. Patients with an upright TaVR were older, had a higher percentage of CVD and hypertension, higher CKMB level, faster heart rate, higher rate of QT prolongation >450 ms, higher rate of negative T in lead II, higher rate of negative T in lead V6, higher rate of ST depression, and longer QTc duration. No significant differences were found between the two groups in regard to percentage of men, diabetes mellitus, hyperlipidemia, incidence of QRS duration >110 ms, levels of K, Na, Ca, CKMB, serum BNP, cTnI, HCY, UA, TG, TC, LDL, and use of ACEI/ARB, beta-blockers, calcium-channel blockers, and statins.
Fig. 1.
Representative aVR lead with an upright T-wave (a) and a negative T-wave (b).
Table 1.
Baseline characteristics of 625 patients.
| Characteristics | Negative T wave | Upright T wave | P value |
|---|---|---|---|
| n,% | 424 (68) | 201 (32) | − |
| Age, yearsa | 64 ± 12 | 69 ± 12 | <0.001 |
| Male, % | 62.7 | 56.2 | 0.120 |
| CVD, % | 7.5 | 33.8 | <0.001 |
| Hypertension, % | 57.8 | 70.1 | 0.003 |
| Diabetes mellitus, % | 22.6 | 27.9 | 0.159 |
| Hyperlipidemia, % | 18.6 | 16.4 | 0.498 |
| ECG findings | |||
| Heart rate, beats/mina | 73 ± 17 | 82 ± 25 | <0.001 |
| QRS duration > 110 ms, % | 8.0 | 12.4 | 0.084 |
| QT prolongation > 450 ms, % | 23.8 | 39.8 | <0.001 |
| Abnormal Q wave, % | 5.7 | 9.0 | 0.133 |
| Negative T wave in lead II, % | 7.8 | 14.9 | 0.013 |
| Negative T wave in lead V6, % | 18.9 | 31.8 | <0.001 |
| ST depression, % | 22.6 | 47.8 | <0.001 |
| QTc, ms | 429.6 ± 45.1 | 438.5 ± 54.5 | 0.032 |
| Laboratory parametersa | |||
| K, mmol/L | 4.0 ± 0.4 | 3.9 ± 0.6 | 0.076 |
| Na, mmol/L | 141.6 ± 3.51 | 141.1 ± 5.0 | 0.108 |
| Ca, mmol/L | 2.4 ± 0.3 | 2.7 ± 0.5 | 0.660 |
| BNP, pg/mL | 7861 ± 6352 | 4155 ± 1329 | 0.612 |
| CKMB, ng/mL | 2.6 ± 1.1 | 9.3 ± 3.4 | 0.035 |
| cTnI, ng/mL | 1.4 ± 1.4 | 1.9 ± 0.8 | 0.783 |
| HCY, ng/mL | 16.3 ± 0.6 | 18.0 ± 1.0 | 0.125 |
| UA, μmol/L | 347.4 ± 5.4 | 362.7 ± 9.2 | 0.128 |
| TC, mmol/L | 4.14 ± 0.05 | 4.25 ± 0.08 | 0.259 |
| TG, mmol/L | 1.60 ± 0.05 | 1.49 ± 0.06 | 0.216 |
| LDL, mmol/L | 2.49 ± 0.04 | 2.56 ± 0.07 | 0.425 |
| Medications use, % | |||
| Aspirin | 42.3 | 38.9 | 0.799 |
| Beta-blocker | 12.3 | 12.6 | 0.951 |
| ACEI/ARB | 32.2 | 36.1 | 0.139 |
| Calcium-channel blocker | 10.8 | 9.6 | 0.590 |
| Statin | 72.5 | 71.4 | 0.374 |
| TOAST stroke subtype, % | |||
| Large-artery atherosclerosis | 50.3 | 54.6 | 0.294 |
| Cardioembolism | 4.2 | 3.5 | 0.646 |
| Lacunar | 27.5 | 24.3 | 0.393 |
| Undetermined | 18.0 | 17.6 | 0.876 |
| All-cause death, % | 1.9 | 10.4 | <0.001 |
| Cardiovascular death, % | 0.7 | 4.5 | 0.002 |
CVD: cardiovascular disease; ECG: Electrocardiography; QTc: rate-corrected QT; BNP: B-type natriuretic peptide; CKMB: creatine kinase MB; cTnI: troponin I; HCY: homocysteine; UA: uric acid; TC: total cholesterol; TG: total glyceride; LDL: low-density lipoprotein cholesterol; ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin II receptor blocker; TOAST: Trial of ORG 10172 in Acute Stroke Treatment classification.
Data are presented as mean ± standard deviation.
No significant differences in neurological presentations were found between the two groups (Table 1). Based on Trial of ORG 10172 in Acute Stroke Treatment classification,11 no significant differences were found in stroke subtype between the two groups (namely large-artery atherosclerosis, lacunar, cardioembolic, and undetermined subtype).
Regarding the mortality between the two groups, the upright TaVR group showed a significantly higher rate of all-cause death (10.4% vs. 1.9%; P < 0.001) and cardiovascular death (4.5% vs. 0.7%; P = 0.002).
Survival analysis
During the mean follow-up period of 20.0 ± 5.8 months, 29 (4.6%) patients experienced all cause death, and of them 12 (1.9%) experienced cardiovascular death. In addition, 94 (15.0%) patients had a recurrence of IS, the secondary end point.
Primary end point
Table 2 summarizes the results of univariate Cox analysis of factors predicting all-cause death. Age, percentage of CVD, heart rate, QT prolongation >450 ms, and the presence of upright TaVR were identified as significant predictors of all-cause death. Multivariate Cox regression analysis revealed that age (HR: 1.07, 95% CI: 1.02–1.11), CVD (HR: 2.42, 95% CI: 1.05–5.59), heart rate (HR: 1.03, 95% CI: 1.02–1.04) and the presence of upright TaVR (HR: 2.88, 95% CI: 1.07–7.73) were independent predictors of all-cause death.
Table 2.
Uni- and multivariate predictors of primary end event.
| Variable | All-cause death |
Cardiovascular death |
||||||
|---|---|---|---|---|---|---|---|---|
| Univariate |
Multivariate |
Univariate |
Multivariate |
|||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Age | 1.08 (1.04–1.12) | <0.01 | 1.07 (1.02–1.11) | <0.01 | 1.05 (1.01–1.10) | 0.02 | 1.2 (0.47–3.51) | 0.47 |
| Male sex | 1.62 (0.72–3.62) | 0.24 | 0.71 (0.27–1.85) | 0.48 | ||||
| CVD | 5.61 (2.46–12.78) | <0.01 | 2.42 (1.05–5.59) | 0.04 | 3.01 (1.20–7.54) | 0.02 | 1.77 (0.66–4.70) | 0.61 |
| Hypertension | 1.72 (0.68–4.36) | 0.26 | 1.34 (0.51–3.53) | 0.56 | ||||
| Heart rate | 1.03 (1.02–1.04) | <0.01 | 1.03 (1.02–1.04) | <0.01 | 1.02 (1.01–1.04) | <0.01 | 1.01 (0.997–1.03) | 0.12 |
| QTc | 0.98 (0.92–1.003) | 0.41 | 1.00 (0.99–1.01) | 0.87 | ||||
| CKMB | 1.00 (0.99–1.01) | 0.57 | 1.00 (0.99–1.02) | 0.43 | ||||
| HCY | 0.97 (0.88–1.06) | 0.51 | 1.00 (0.95–1.06) | 0.93 | ||||
| Statin use | 0.63 (0.28–1.41) | 0.26 | 1.46 (0.53–4.03) | 0.47 | ||||
| QT prolongation > 450 ms | 2.28 (1.02–5.08) | 0.04 | 1.09 (0.45–2.65) | 0.85 | 4.36 (1.73–10.97) | 0.04 | 2.76 (1.05–7.22) | 0.04 |
| Negative T in lead II | 1.50 (0.49–4.57) | 0.48 | 1.92 (0.62–5.92) | 0.35 | ||||
| Negative T in lead V6 | 1.76 (0.78–3.97) | 0.18 | 1.94 (0.79–4.76) | 0.15 | ||||
| ST depression | 1.19 (0.51–2.79) | 0.69 | 1.76 (0.73–4.24) | 0.21 | ||||
| Upright TaVR | 6.10 (2.39–15.53) | <0.01 | 2.88 (1.07–7.73) | 0.04 | 4.65 (1.77–12.24) | <0.01 | 3.04 (1.07–8.64) | 0.04 |
Parameters with a P value < 0.05 were entered in the multivariate analysis.
Upright TaVR was defined as T-wave with a positive deflection ≥ 0 mV in lead aVR.
HR: hazard ratio; CI: confidence interval; CVD: cardiovascular disease; QTc: rate-corrected QT; CKMB: creatine kinase MB; HCY: homocysteine; TaVR: T wave in lead aVR.
Table 2 also summarizes the results of univariate Cox analysis of factors predicting cardiovascular death. Age, percentage of CVD, heart rate, QT prolongation >450 ms, and the presence of upright TaVR were identified as significant predictors of cardiovascular death. Multivariate Cox regression analysis revealed that QT prolongation >450 ms (HR: 2.76, 95% CI: 1.05–7.22) and upright TaVR (HR: 3.04, 95% CI: 1.07–8.64) were independent predictors of cardiovascular death.
Secondary end point
Table 3 shows the results of the univariate and multivariate Cox proportional hazards regression analyses. Univariate regression analyses showed that age, percentage of CVD, heart rate, QT prolongation >450 ms, negative T in lead V6, and upright TaVR were significantly associated with the secondary end point. Multivariate regression analysis showed that age (HR: 1.02, 95% CI: 1.00–1.05), heart rate (HR: 1.02, 95% CI: 1.00–1.03), negative T in lead V6 (HR: 2.12, 95% CI: 1.22–3.68) and upright TaVR (HR: 1.86, 95% CI: 1.08–3.20) were independent predictors of IS recurrence.
Table 3.
Uni- and multivariate predictors of secondary end event.
| Variable | Univariate |
Multivariate |
||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age | 1.02 (1.00–1.04) | 0.01 | 1.02 (1.00–1.05) | 0.03 |
| Male sex | 1.19 (0.76–1.87) | 0.44 | ||
| CVD | 1.67 (1.06–2.65) | 0.03 | 1.19 (0.63–2.26) | 0.59 |
| Hypertension | 1.133 (0.72–1.78) | 0.59 | ||
| Heart rate | 1.02 (1.01–1.03) | <0.01 | 1.02 (1.00–1.03) | <0.01 |
| QTc | 1.00 (0.998–1.01) | 0.16 | ||
| CKMB | 0.98 (0.92–1.05) | 0.61 | ||
| HCY | 1.02 (0.99–1.06) | 0.24 | ||
| Statin | 0.89 (0.59–1.33) | 0.57 | ||
| QT prolongation > 450 ms | 1.80 (1.11–2.92) | 0.02 | 1.31 (0.78–2.20) | 0.30 |
| Negative T in lead II | 1.62 (0.59–4.48) | 0.35 | ||
| Negative T in lead V6 | 2.39 (1.44–3.97) | <0.01 | 2.12 (1.22–3.68) | <0.01 |
| ST depression | 1.18 (0.71–1.96) | 0.52 | ||
| Upright TaVR | 2.27 (1.50–3.43) | <0.01 | 1.86 (1.08–3.20) | 0.03 |
Parameters with a P < 0.05 were entered in the multivariate analysis.
Upright TaVR was defined as T-wave with a positive deflection ≥ 0 mV in lead aVR.
HR: hazard ratio; CI: confidence interval; CVD: cardiovascular disease; QTc: rate-corrected QT; CKMB: creatine kinase MB; HCY: homocysteine; TaVR: T wave in lead aVR.
Discussion
This study found for the first time that the upright TaVR is a significant independent predictor of death or recurrence of IS in patients with IS, after adjusting for different risk factors, such as age, CVD, heart rate, etc. This result implies that the standard resting ECG to detect an upright TaVR may be a useful procedure for predicting death or recurrence of IS in patients with IS.
At baseline, the prevalence of upright TaVR in the study population was 32.2%, which was higher than that in previous studies. In the general population, an upright TaVR was observed in 2.2% of people,5 and other studies showed that the prevalence of upright TaVR was 16.2% in patients with a prior MI9 and 17.5% in patients with heart failure.7 The prevalence of upright TaVR would increase with aging. A study showed that the prevalence of T wave abnormalities in general increases with age, being 5.9% at 50 years of age and 16.0% at 70 years of age.5 Additionally, we defined upright TaVR as a positive deflection ≥0 mV in lead aVR, which also included flat TaVR (amplitude = 0 mV). Therefore, the prevalence of upright TaVR in this study may be higher than that in previous studies,4, 9 which did not include flat TaVR.
It has been reported that TaVR is associated with increased risk of cardiovascular death and sudden cardiac death. In the present study, we also found that upright TaVR increased risk of cardiovascular death (HR: 3.04, 95% CI: 1.07–8.64). The mechanism for upright TaVR leading to an increased risk for mortality is currently speculative. Lead aVR, which gives information from the right upper side of the heart, has been considered to provide reciprocal information from the left lateral side of the heart, which is already covered by leads aVL, II, V5, and V6.12 When repolarization of injured myocardial cells is delayed compared to normal cells, the direction of the T wave vector changes towards the injured myocardial region.13 Several studies have revealed that a long left anterior descending artery, multivessel disease, and is chemically injured myocardium in above-mentioned areas of the heart would be expected to invert a normally negative TaVR and lead to an upright T wave.14, 15 This theory supports ischemia as a cause of repolarization abnormalities leading to upright TaVR.8
IS and CVD share some common pathophysiological mechanisms (i.e., atherosclerotic) and risk factors.16 The risk of cardiovascular events is increased after IS. American Heart Association and American Stroke Association recommend an individual risk assessment based mainly on the score risk to identify patients with the highest likelihood of morbidity and mortality from unrecognized CVD after a stroke.17 Several studies have suggested that 25–60% of patients with stroke and without clinical manifestations of CVD may have a relatively high risk of silent myocardial ischemia and non-stroke vascular death,17, 18 while one study reported that about one third of patients (including patients with stroke) evaluated before carotid surgery had ≥ 1 coronary artery stenosis ≥70%.19 Although the mechanism by which an upright TaVR increases the risk of mortality or recurrence of IS in patients with IS remains unknown, it could be speculated that coronary atherosclerotic lesions may occur after IS and can lead to upright TaVR due to coronary artery stenosis. More researches are needed to identify the association between upright TaVR and increased risk of mortality or recurrence rate in patients with IS.
Our study has several limitations. First, we have no data to support proposed explanations regarding the underlying mechanism. Second, the exclusion of patients with some clinical conditions potentially associated with a higher mortality, such as atrial fibrillation, may also add bias to obtained results. Third, the mortality of patients with IS is influenced by multiple risk factors; therefore, a comprehensive evaluation, rather than single measurements of TaVR, is necessary to assess an increased mortality risk, making TaVR a useful predictor of clinical events in patients with IS. Fourth, short-term follow-ups of this study have been performed. A longer follow-up period may have provided additional data.
Conclusions
This study showed that upright TaVR is associated with an increased risk of death and IS recurrence in patients with IS.
Conflicts of interest
All authors declare no conflicts of interest.
Acknowledgement
This work was supported by grant 2014CFA061 from the Key Foundation of Hubei Province of China.
Edited by Jing-Ling Bao
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Khechinashvili G., Asplund K. Electrocardiographic changes in patients with acute stroke: a systematic review. Cerebrovasc Dis. 2002;14:67–76. doi: 10.1159/000064733. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein D.S. The electrocardiogram in stroke: relationship to pathophysiological type and comparison with prior tracings. Stroke. 1979;10:253–259. doi: 10.1161/01.str.10.3.253. [DOI] [PubMed] [Google Scholar]
- 3.Christensen H., Fogh C.A., Boysen G. Abnormalities on ECG and telemetry predict stroke outcome at 3 months. J Neurol Sci. 2005;234:99–103. doi: 10.1016/j.jns.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 4.Tan S.Y., Engel G., Myers J., Sandri M., Froelicher V.F. The prognostic value of T wave amplitude in lead aVR in males. Ann Noninvasive Electrocardiol. 2008;13:113–119. doi: 10.1111/j.1542-474X.2008.00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anttila I., Nikus K., Nieminen T. Relation of positive T wave in lead aVR to risk of cardiovascular mortality. Am J Cardiol. 2011;108:1735–1740. doi: 10.1016/j.amjcard.2011.07.042. [DOI] [PubMed] [Google Scholar]
- 6.Badheka A.O., Patel N.J., Grover P.M. ST-T wave abnormality in lead aVR and reclassification of cardiovascular risk (from the National Health and Nutrition Examination Survey-III) Am J Cardiol. 2013;112:805–810. doi: 10.1016/j.amjcard.2013.04.058. [DOI] [PubMed] [Google Scholar]
- 7.Okuda K., Watanabe E., Sano K. Prognostic significance of T-wave amplitude in lead aVR in heart failure patients with narrow QRS complexes. Ann Noninvasive Electrocardiol. 2011;16:250–257. doi: 10.1111/j.1542-474X.2011.00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shinozaki K., Tamura A., Kadota J. Associations of positive T wave in lead aVR with hemodynamic, coronary, and left ventricular angiographic findings in anterior wall old myocardial infarction. J Cardiol. 2011;57:160–164. doi: 10.1016/j.jjcc.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Torigoe K., Tamura A., Kawano Y., Shinozaki K., Kotoku M., Kadota J. Upright T waves in lead aVR are associated with cardiac death or hospitalization for heart failure in patients with a prior myocardial infarction. Heart Ves. 2012;27:548–552. doi: 10.1007/s00380-011-0193-6. [DOI] [PubMed] [Google Scholar]
- 10.Ayhan E., Isık T., Uyarel H. Prognostic significance of T-wave amplitude in lead aVR on the admission electrocardiography in patients with anterior wall ST-elevation myocardial infarction treated by primary percutaneous intervention. Ann Noninvasive Electrocardiol. 2013;18:51–57. doi: 10.1111/j.1542-474X.2012.00530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adams H.P., Bendixen B.H., Kappelle L.J. Classifiscation of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 12.Gorgels A.P., Engelen D.J., Wellens H.J. Lead aVR, a mostly ignored but very valuable lead in clinical electrocardiography. J Am Coll Cardiol. 2001;38:1355–1356. doi: 10.1016/s0735-1097(01)01564-9. [DOI] [PubMed] [Google Scholar]
- 13.Rautaharju P.M., Surawicz B., Gettes L.S. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval: a scientific statement from the American heart association electrocardiography and arrhythmias committee, council on clinical cardiology; the American college of cardiology foundation; and the heart rhythm society. Endorsed by the international society for computerized electrocardiology. J Am Coll Cardiol. 2009;53:982–991. doi: 10.1016/j.jacc.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 14.Tamura A. Significance of lead aVR in acute coronary syndrome. World J Cardiol. 2014;6:630–637. doi: 10.4330/wjc.v6.i7.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.George A., Arumugham P.S., Figueredo V.M. aVR - the forgotten lead. Exp Clin Cardiol. 2010;15:e36–44. [PMC free article] [PubMed] [Google Scholar]
- 16.Pasternak R.C., Criqui M.H., Benjamin E.J. Atherosclerotic vascular disease conference: writing group I: epidemiology. Circulation. 2004;109:2605–2612. doi: 10.1161/01.CIR.0000128518.26834.93. [DOI] [PubMed] [Google Scholar]
- 17.Adams R.J., Chimowitz M.I., Alpert J.S. Coronary risk evaluation in patients with transient ischemic attack and ischemic stroke: a scientific statement for healthcare professionals from the stroke council and the council on clinical cardiology of the American heart association/American stroke association. Circulation. 2003;108:1278–1290. doi: 10.1161/01.CIR.0000090444.87006.CF. [DOI] [PubMed] [Google Scholar]
- 18.Touzé E., Varenne O., Chatellier G., Peyrard S., Rothwell P.M., Mas J.L. Risk of myocardial infarction and vascular death after transient ischemic attack and ischemic stroke: a systematic review and meta-analysis. Stroke. 2005;36:2748–2755. doi: 10.1161/01.STR.0000190118.02275.33. [DOI] [PubMed] [Google Scholar]
- 19.Hertzer N.R., Young J.R., Beven E.G. Coronary angiography in 506 patients with extracranial cerebrovascular disease. Arch Intern Med. 1985;145:849–852. [PubMed] [Google Scholar]