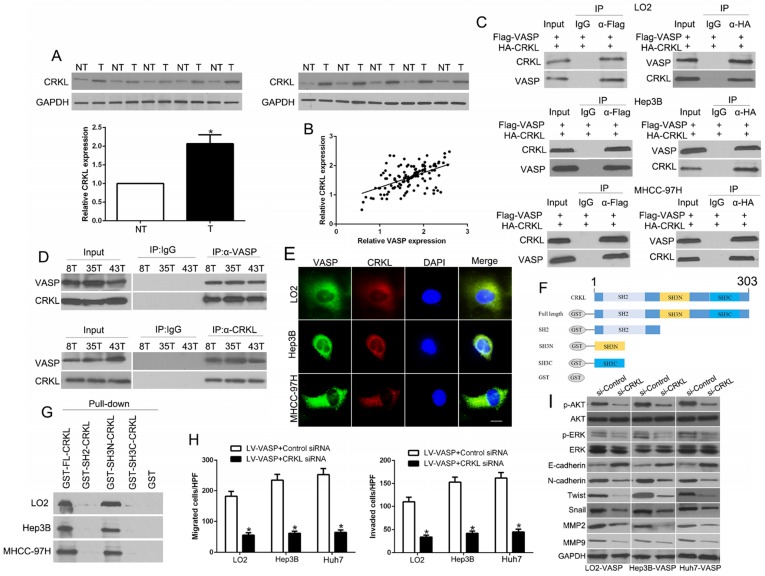
Figure 5.
The N-terminal SH3 domain of CRKL dynamically interacts with VASP and mediates its functional effects. (A) Expression of CRKL in paired human HCC and matched adjacent non-tumorous tissues. (B) Correlation between VASP and CRKL in human HCC tissues. (C) Co-IP analysis of the interaction between VASP protein and CRKL protein in HCC cells. (D) Co-IP analysis of the interaction between VASP protein and CRKL protein in HCC tissues. (E) Confocal images of LO2, Hep3B, and MHCC-97H cells stained for CRKL (red) and VASP (green) and 4′,6-diamidino-2-phenylindole (DAPI; blue). Colocalization was indicated by the merged images showing yellow immunofluorescence. (F) Schematic diagram of the CRKL domain organization and the different GST-CRKL fusion proteins used in this study. (G) GST pull-down assay with lysates of human cells. Equal amounts of lysates were incubated with equimolar amounts of the depicted, immobilized GST fusion proteins or GST alone. After extensive washing, precipitated material was analyzed by Western blotting with anti-VASP antibodies. Cells overexpressing VASP and corresponding cells in the control group were transfected with CRKL siRNA or control siRNA. 48 h after transfection, cells were subjected to the Transwell assay for migration and invasion (H) and Western blotting for EMT markers (I). n = six independent experiments.