Figure 4.
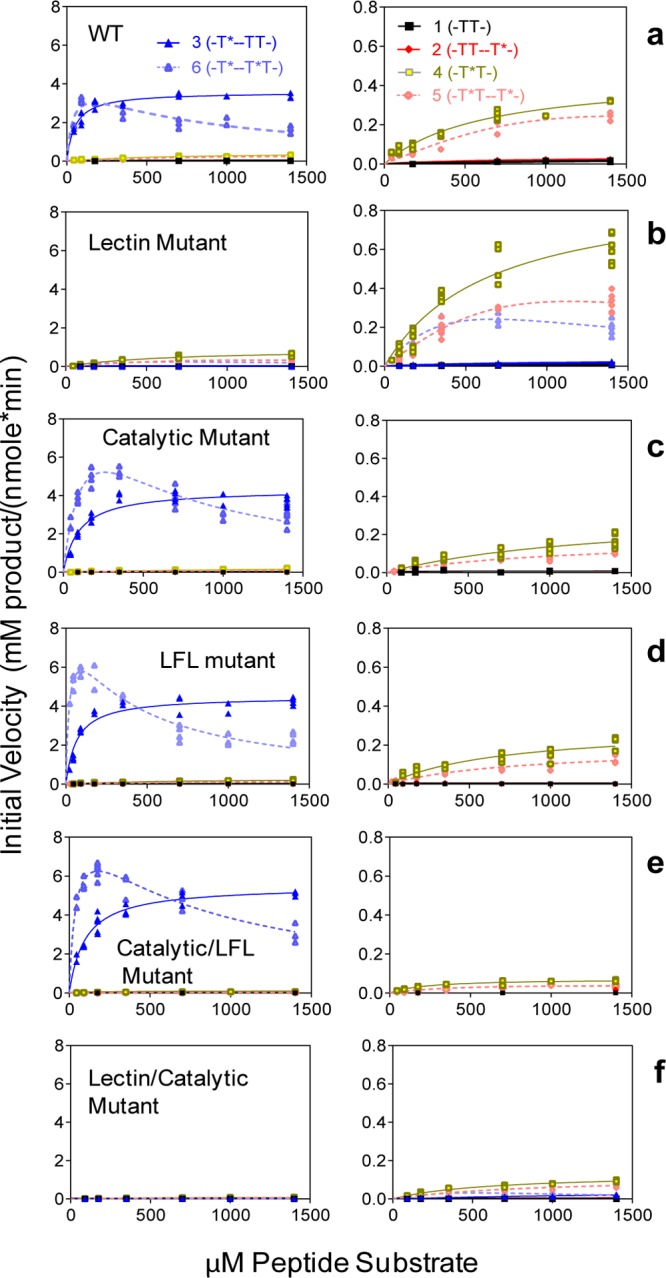
Enzyme kinetics of wt and mutant GalNAc-T4 against the (glyco)peptide substrates in Table 1. Note that the left and right panels are plotted with different initial activity scales. Kinetic constants obtained from the plots are given in Table 2. (a) Wild-type GalNAc-T4 showing both long-range (left panel) and short-range (right panel) glycopeptide activities. (b) Lectin mutant (D549H) showing the loss of its long-range glycopeptide activity. (c) Catalytic mutant (T283S, Q285A) showing the partial loss of GalANc-T4’s short-range prior glycopeptide activity. (d) Lectin flexible link (LFL) mutant (P463DNNP467 to GGG) showing a partial loss of the short-range glycopeptide activity while possessing an intact catalytic domain. (e) Catalytic/LFL combined mutant (T283S, Q285A, D464A) showing a more complete loss of the short-range glycopeptide activity. (f) Lectin/catalytic combined mutant (T283S, Q285A, D459H) showing the near complete loss of both the long-range and short-range glycopeptide activities.
