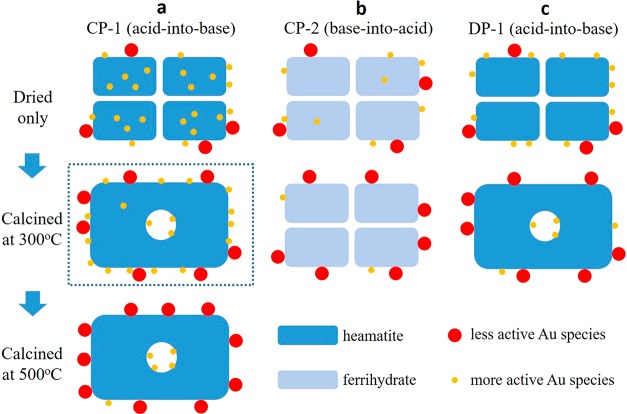
Figure 3.
Proposed mechanism for the thermal activation behavior of the CP-1 catalyst (reproduced from ref (13)). A series of schematic diagrams which illustrate the thermal evolution process of the (a) CP-1 (acid-into-base), (b) CP-2 (base-into-acid), and (c) DP-1 (acid-into-base) catalysts. The CP-1 catalyst (column a) has a much larger amount of atomic Au species buried inside the support material after only being dried as compared to the CP-2 and DP-1 catalysts (columns b and c, respectively). Therefore, after calcination at 300 °C, the loss of the more active smaller Au species (i.e., sub-nm clusters and 1–3 nm Au particles) due to agglomeration can be replenished in the CP-1 catalysts by the outward diffusion of the “trapped” internal Au species, which is not possible in the case of the CP-2 and DP-1 catalysts. As a result, the CP-1 catalyst after calcination at 300 °C can be even more active than the dried only stage (as highlighted by the dashed box). However, after prolonged calcination at higher temperatures (i.e., the 500 °C treatment) the Au reserves inside the CP-1 support particle eventually get depleted, and the catalytic activity decreases close to zero due to agglomeration of the surface Au species.