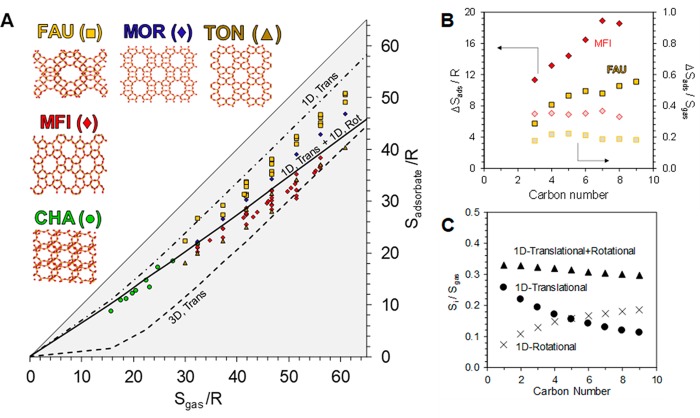
Figure 2.
(A) Comparison of adsorbate and gas-phase entropy in MFI, CHA, TON, FAU, and MOR. The gray triangle indicates the entropy region that is thermodynamically accessible; the entropy of the adsorbate cannot exceed what is available in the gas phase. (B) Entropy of adsorption of linear alkanes on MFI and FAU. Absolute values of the entropy of adsorption and those normalized by gas-phase entropy are indicated by filled and open symbols, respectively. (C) Entropy associated with one degree of translational and rotational movement, as well as the sum of the two modes as a fraction of the total gas-phase entropy for linear alkanes.