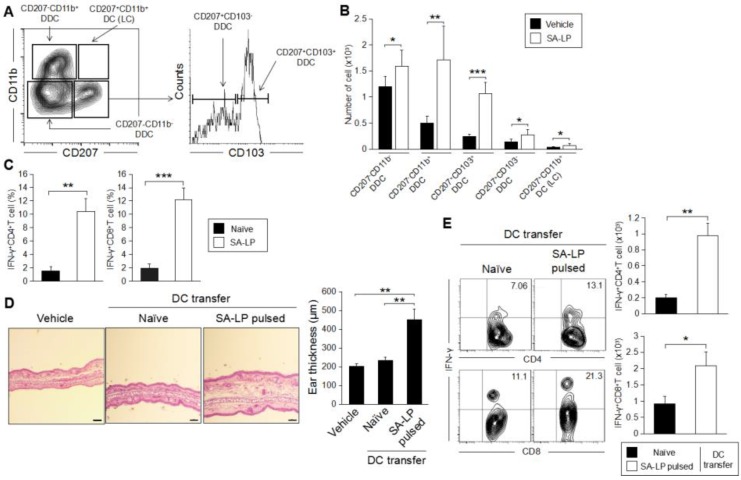
Figure 5.
SA-LP-activated dermal dendritic cells have the responsibility of inducing skin inflammation. (A) The gating strategy of skin DC subset analysis. The mDC (MHC class IIhiCD11c+) population was gated, then the population was separated to each subset by following CD207 and CD11b expression. The CD207+CD11b− population was further separated with CD103 expression. Finally, skin DCs were classified as five subsets (CD207−CD11b−, CD207−CD11b+, CD207+CD103+, CD207+CD103−, and CD207+CD11b+ (Langerhans cell; LC)). (B) Activated DC subset classification on the SA-LP ID-injected mice. WT mice were treated with SA-LP (10 μg) or vehicle solution (0.9% NaCl) by ID injection into the ear. After 48 hr of treatment, the skin-dLN cells were isolated, and stained with anti-MHCII, anti-CD11c, anti-CD11b, anti-CD207, and anti-CD103 mAb. The stained cells were analyzed by flow cytometry by following the gating strategy (A). (C) Ex vivo antigen presentation for naive T cells. The DCs were isolated from skin-dLN in the SA-LP ID-injected mice. The DCs were cocultured with splenic naive T cells for 72 hr. For the last 6 hr, the proliferated cells were re-stimulated with PMA (100 ng/mL) and ionomycin (1 μg/mL). The cells were extracellularly and intracellularly stained for the detection of IFN-γ+CD4+, and CD8+T cells in the population gated for CD3+ cells by flow cytometry. (D,E) SA-LP-activated DC transfer induced skin inflammation bearing IFN-γ-producing T cells. The DCs were isolated from LN, and stimulated with SA-LP for 24 hr. The DCs were transferred into WT mice ears by ID injection. After 120 hr of treatment, the mice were used for analysis. (D) Skin histology of the treated ear. Ear section stained with H-E. The scale bar represents 100 μm. Thickness of the treated ear was measured from each section. (E) Subset analysis for accumulated T cells on the inflamed skin. Skin leukocytes were isolated from the ear, then the cells were re-stimulated with PMA (100 ng/mL) and ionomycin (1 μg/mL) for 6 hr. The stimulated cells were extracellularly and intracellularly stained for detection of IFN-γ+ CD4+ and CD8+T cells in the population gated for CD3+ cells by flow cytometry. The total number of target cells was calculated from the flow cytometry image. (B,E) In each experiment, 3–5 mice were used for vehicle and SA-LP ID injection. The isolated cells from the ear and skin-dLN were pooled, and used for flow cytometry analysis. Data are shown as the mean and SD of at least three samples of a single experiment, and are representative of at least three independent experiments. A Student’s t-test was used to analyze data for significant differences. Values of * p < 0.05, ** p < 0.01 and *** p < 0.001 were regarded as significant.