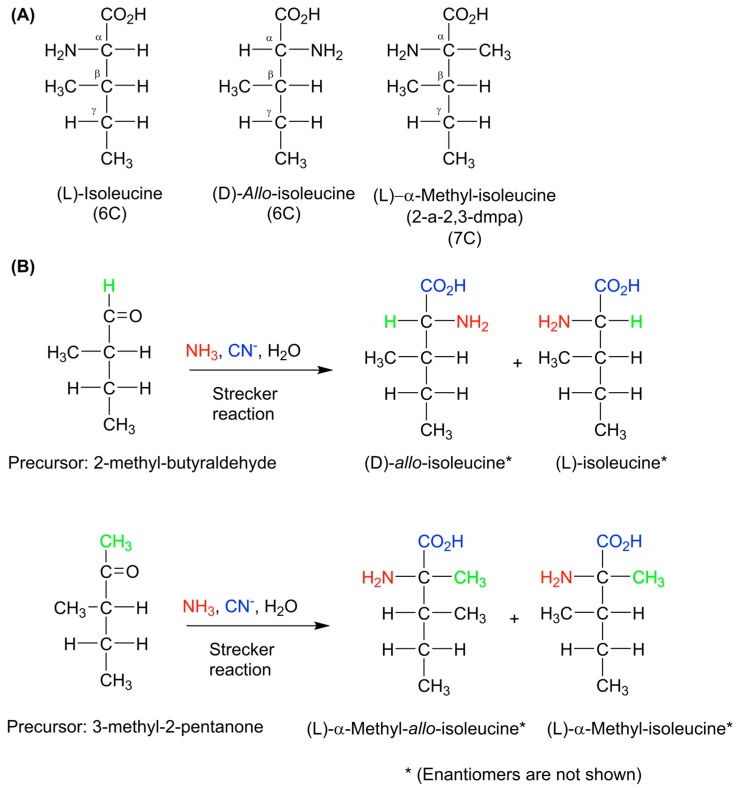
Scheme 2.
(A) The structural relationship between isoleucine, allo-isoleucine and α-methyl-isoleucine; (B) An illustration of obtaining specific diastereomers in the same Strecker reaction. Each diastereomer has its own enantiomer ratio. From the structures of allo-isoleucine and isoleucine it can be seen that they are not mirror images (enantiomers) of each other. Instead, they are epimers, i.e., stereoisomers that have multiple chiral centers, but only differ from one another by the configuration at one of the chiral centers. It is much easier to epimerize either compound into the other by inverting the stereochemistry at the α carbon because it contains an exchangeable hydrogen. However it is much more difficult to invert both α and β carbons of a given compound to obtain its enantiomer (see text).