Figure 4.
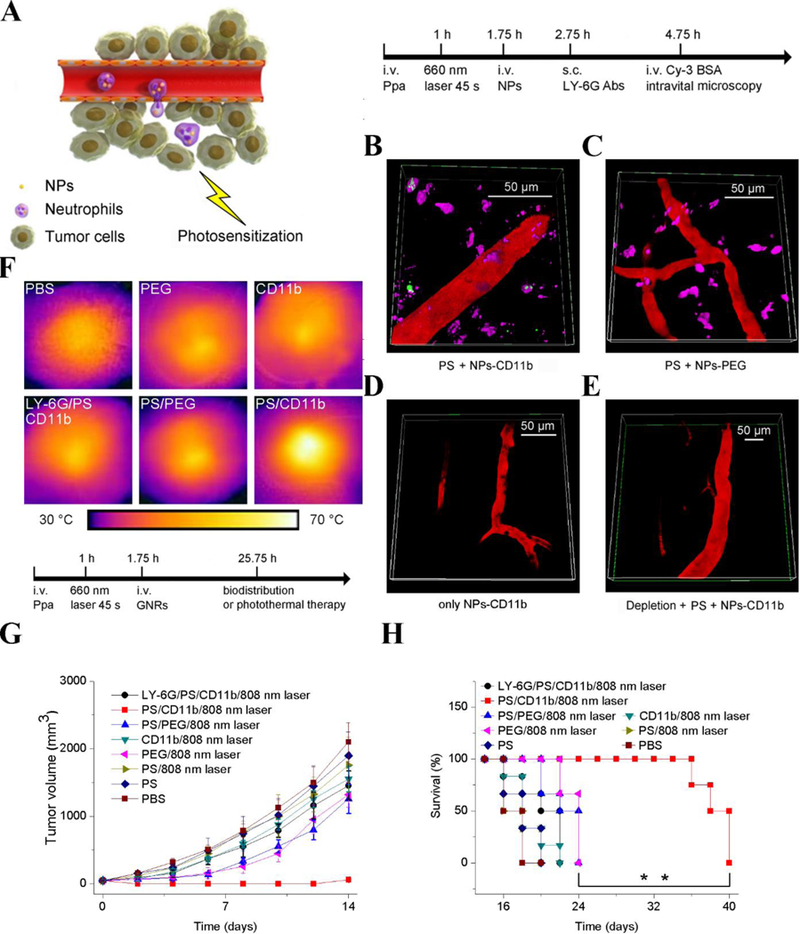
Neutrophil-mediated delivery of NPs to tumors across blood vessel walls. (A) The concept of neutrophil-mediated delivery of NPs to tumor tissues inflamed by photosensitization (PS). Intravital microscopic 3D images of mouse tumors treated with (B) both PS and NPs-CD11b (green), (C) PS and NPs-PEG (green), (D) NPs-CD11b only and (E) PS and NPs-CD11b after neutrophil depletion. The tumor was treated with PS (intravenous (i.v.) injection of pyropheophorbide-a (Ppa) and 660-nm laser). Approximately 0.75 h later, NPs were i.v. administered. LY-6G Abs (pink) was subcutaneously (s.c.) injected around the tumor to stain neutrophils. Cy3-BSA (red) was i.v. administered to visualize the blood vessel. Anti-LY-6G Abs were intraperitoneally (i.p.) injected 24 h before the administration of Ppa to deplete microphils. (F) Thermal graphic imaging of mouse tumors after the tumors were irradiated with a laser for 20 min. (G) Tumor size and (H) survival rate of the tumor-bearing mice. The mice were irradiated with an 808-nm laser after the injection of GNRs-PEG and GNRs-CD11b with or without PS (denoted as PS/PEG, PS/CD11b, PEG, and CD11b, respectively) or irradiated with an 808-nm laser after the treatment of both PS and GNRs-CD11b with neutrophil depletion (denoted as LY-6G/PS/CD11b). For PBS (without PS or laser irradiation at 808 nm), PS (with PS but without laser irradiation at 808 nm), and PS/808 nm laser (with both PS and laser irradiation at 808 nm), only PBS (pH 7.4) was injected. Data represent mean ± SD (n = 6 mice per group). Copyright 2017, Wiley.[13]
