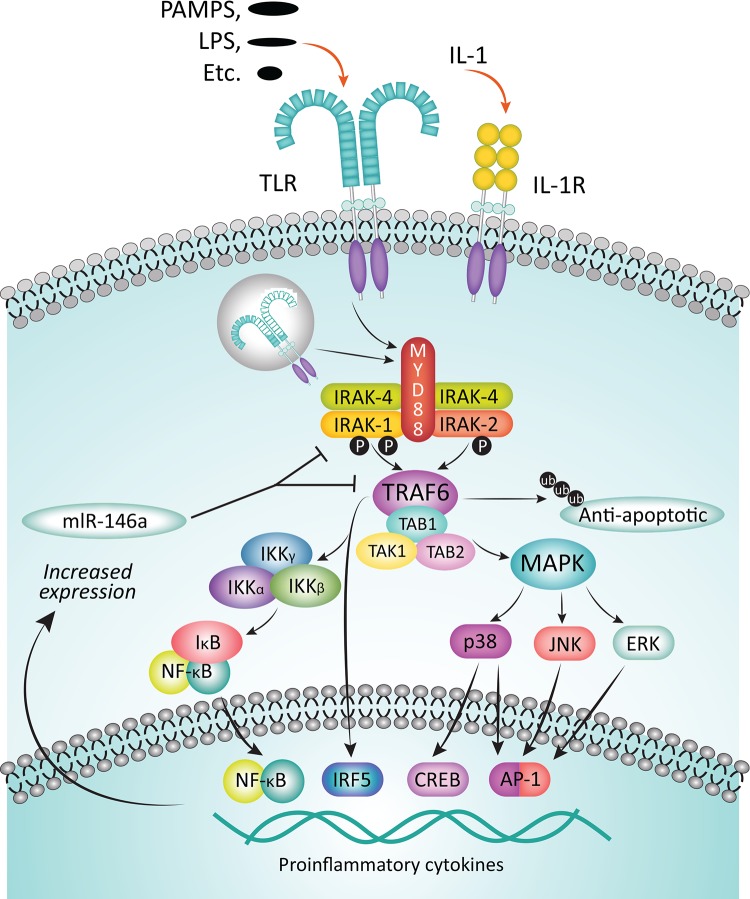
Figure 2. Function of IRAKs in the myddosome complex.
Upon binding of cognate ligands, such as PAMPs and LPS, to TLRs or of IL-1 to the IL-1R, inflammatory response is mediated via the myddosome complex. Adapter protein MyD88 is recruited to the cytosolic receptor domain and IRAK4 is recruited, in turn attracting IRAK1 to the complex. IRAK4 phosphorylates IRAK1, thereby activating it, leading to its subsequent hyperphosphorylation, dissociation from the complex, and binding to TRAF6. The activated TRAF6 complex drives downstream gene transcription via multiple pathways, including the NF-kB pathway. Among the sequelae are an increased expression of inflammatory cytokines, but also miR-146a, which inhibits subsequent expression of IRAK-1 and TRAF6 proteins, thereby providing a negative feedback loop. Adapted from Jain A, Kaczanowska S, Davila E. IL-1 receptor-associated kinase signaling and its role in inflammation, cancer progression, and therapy resistance. Front Immunol. 2014;5:553-561. Used with permission.