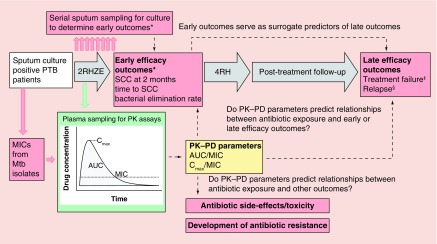
Figure 1. . Generic study design for clinical PK–PD study in DS-TB.
Procedures to generate PK parameters are shown in green. Procedures to generate PD parameters and study outcome measures are shown in red. PK–PD parameters which may be related to study outcome measures are shown in yellow.
Number before drug combinations denotes intended duration of therapy in months. Other abbreviations are as described in the main text.
*TB cultures to determine early efficacy measures may be set up in solid or liquid culture.
‡Treatment failure is normally defined as persistently positive sputum cultures until the end of TB therapy.
§Relapse is normally defined as cure (negative sputum culture) at the end of TB therapy, but reversion to positive cultures with the same Mtb strain as the baseline isolate during post-treatment follow-up.
E: Ethambutol; H: Isoniazid; MIC: Minimum inhibitory concentration; Mtb: Mycobacterium tuberculosis; PTB: Pulmonary tuberculosis; R: Rifampicin; SCC: Sputum culture conversion; Z: Pyrazinamide.