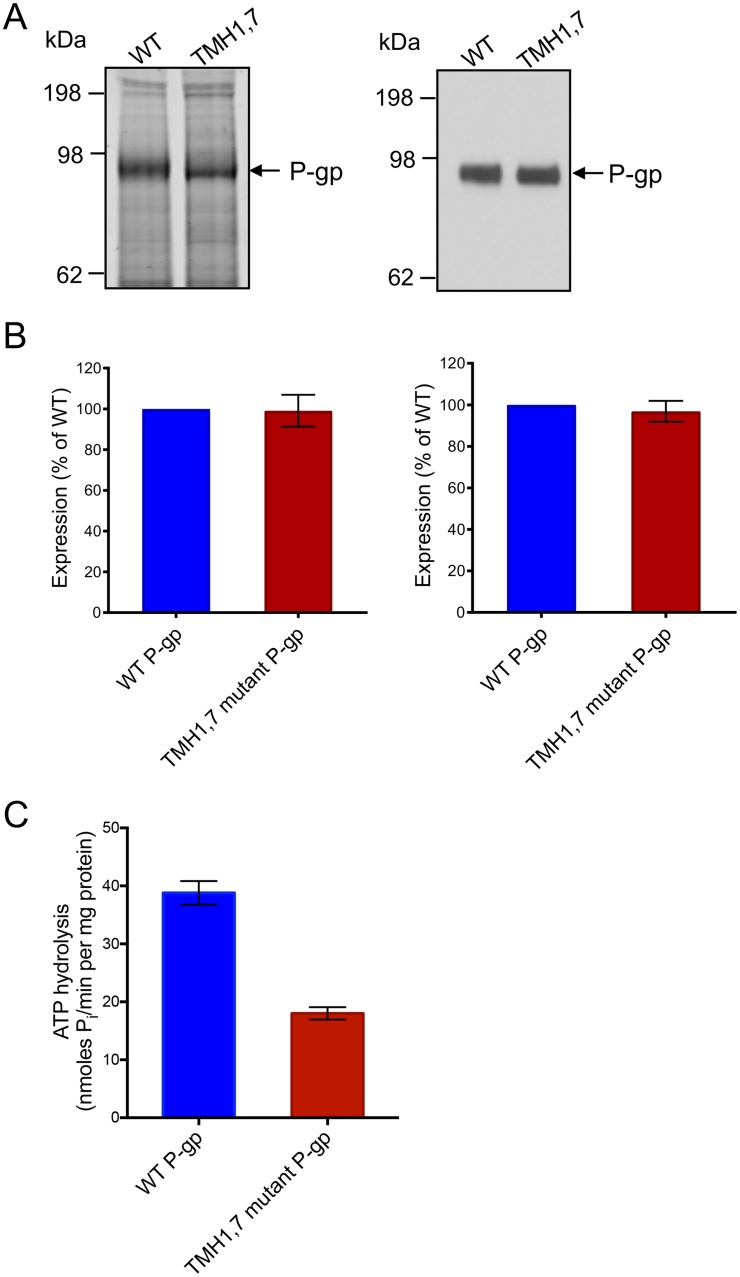
Fig 5. The basal ATPase activity of the TMH1,7 mutant is lower than WT P-gp despite similar expression in insect cell membranes.
TMH1,7 mutant and WT P-gp membrane vesicles were prepared from High-Five insect cells by hypotonic lysis and differential centrifugation, as described in “Materials and Methods”. (A) 10 μg of total protein of membrane vesicles per lane was used for SDS-PAGE on 7% Tris-acetate gel (left) and 1 μg of total protein was used for Western blotting with C-219 antibody (right). (B) Bar graphs showing quantification of the SDS-PAGE gel using Licor Odyssey (left) and Western blot using Fiji-imageJ (right). The values represent the mean from three to five independent experiments and error bars show SD. (C) ATP hydrolysis was measured in the presence and absence of 0.3 mM sodium orthovanadate in membrane vesicles expressing WT or TMH1,7 mutant P-gp. Membranes (10 μg protein per 100 μL reaction volume) were incubated in ATPase assay buffer and vanadate-sensitive activity was measured as described previously [39]. The basal ATPase activity of TMH1,7 mutant P-gp was compared with WT P-gp. The histogram shows mean values from five independent assays in duplicate and error bars indicate SD.