Abstract
Cocaine use disorder is a serious public health issue for which there is no effective pharmacotherapy. One strategy to speed development of medications for cocaine use disorder is to repurpose drugs already approved for use in humans based on their ability to interact with targets known to be important for addiction. Two such drugs, lorcaserin (Belviq®; a drug with serotonin [5-HT]2C receptor agonist properties), and buspirone (Buspar®; a drug with 5-HT1A receptor partial agonist and dopamine D3/D4 receptor antagonist properties) can produce modest decreases in cocaine self-administration in rhesus monkeys. The current study evaluated the effectiveness of mixtures of lorcaserin and buspirone, (at fixed dose ratios of 3:1, 1:1, and 1:3 relative to each drug’s ID50), to reduce responding for 0.032 mg/kg/inf cocaine under a progressive ratio schedule of reinforcement in 2 male and 2 female rhesus monkeys. Dose addition analyses were used to determine if the effects of the drug mixtures differed from those predicted for an additive interaction between lorcaserin and buspirone. Dose-dependent reductions of cocaine self-administration were observed when lorcaserin and buspirone were administered alone, as well as when they were administered as 3:1, 1:1, and 1:3 fixed ratio mixtures of lorcaserin + buspirone. The effects of the 1:1 mixture of lorcaserin + buspirone on cocaine self-administration were supra-additive, whereas the effects of 3:1 and 1:3 mixtures were additive. Together, these results indicate that a combination therapy containing a mixture of lorcaserin and buspirone might be more effective than either drug alone at treating cocaine use disorder.
Keywords: cocaine self-administration, lorcaseirn, buspirone, rhesus monkeys
Introduction
Cocaine use disorder remains a significant public health problem, with recent worldwide estimates suggesting that there are approximately 20 million regular users of cocaine (United Nations Office on Drugs and Crime, 2016). Despite longstanding efforts to develop pharmacotherapies, the US Food and Drug Administration (FDA) has yet to approve a medication for treating cocaine use disorder. This is likely due, at least in part, to the indirect mechanisms of action of cocaine (i.e., inhibition of dopamine, serotonin (5-HT), and norepinephrine uptake). However, because dopamine is thought to play a central role in the abuse-related effects of cocaine and other cocaine-like drugs (e.g., Ritz et al., 1987; Thomsen et al., 2009; Gannon et al., 2017), three basic strategies have been employed for developing pharmacotherapies for cocaine abuse: 1) direct or indirect dopamine receptor agonists, which aim to provide a replacement drug for cocaine (e.g., Grabowski et al., 2004; Negus & Henningfield, 2015); 2) cocaine antagonists, which aim to block cocaine at its site(s) of action (e.g., Newman et al., 2005; Rothman et al., 2008); and 3) modulators of cocaine, which aim to alter the effects of cocaine through actions at sites other than the dopamine transporter (DAT) or dopamine receptors (e.g., Mello, 1990; Roberts & Brebner, 2000; Platt et al., 2002; Dackis & O’Brien, 2003). Although such rational approaches to drug development have the potential to be truly transformative, they come with a substantial and ever growing price tag. Recent estimates from the Tufts Center for the Study of Drug Development suggest that a new drug requires ~10 years and a $2.8 billion investment in research and development to obtain FDA approval (DiMasi et al., 2016).
One strategy to reduce the time and cost associated with developing pharmacotherapies for addiction has been to rationally repurpose drugs already FDA-approved for other indications. By selecting drugs based on their capacity to engage biologic targets already known to be important (i.e., neural substrates of addiction), such an approach dramatically reduces the time and cost associated with getting a candidate medication in the clinic. One such target is the 5-HT2C receptor, which is known to play an important role in modulating goal-directed behaviors, such as feeding (Tecott et al., 1995; Vickers et al., 1999). These receptors are highly expressed on dopamine and GABA neurons within the ventral tegmental area (VTA) and nucleus accumbens (NAcc), and play important roles in controlling the activity of dopamine systems, including modulating cocaine-induced increases in dopamine within the NAcc (Alex & Pehek, 2007; Bubar & Cunningham, 2008; Navailles et al., 2008; Howell & Cunningham, 2015). Consistent with their capacity to inhibit dopaminergic responses to cocaine, a variety of 5-HT2C receptor-preferring agonists (e.g., Ro 60-0175, MK 212, mCPP, WAY-163909, and CP-809,101) have been shown to decrease cocaine self-administration (Grottick et al., 2000; Fletcher et al., 2004; 2008; 2010; Cunningham et al., 2011). Recently, we and others have shown that lorcaserin (Beliviq®), a 5-HT2C receptor-preferring agonist approved by the FDA for treating obesity, can reduce cocaine self-administration following both acute and repeated administrations (Collins et al., 2016a; Gerak et al., 2016; Harvey-Lewis et al., 2016; Gannon et al., 2018a; but see Banks & Negus, 2017), suggesting that it may have clinical utility for treating cocaine use disorder. However, it should be noted that doses of lorcaserin only slightly larger than those approved/required to produce its therapeutic effects have been reported to producing feelings of “high” and “hallucinations” in humans (Shram et al., 2011), and to induce head-twitching in rodents (Serafine et al., 2015), both of which are consistent with agonist actions at 5-HT2A receptors.
Based in large part on their relatively restricted pattern of distribution within limbic structures (Levesque et al., 1992; Guerevich & Joyce, 1999), dopamine D3 receptors have also been suggested as viable targets for treating cocaine use disorder (Heidbreder, 2008; Newman et al., 2005; Newman et al., 2012). Buspirone (Buspar®) is a 5-HT1A receptor-preferring partial agonist that is approved by the FDA for treating of anxiety; however, recent studies suggest that buspirone is roughly equipotent at 5-HT1A, dopamine D3, and D4 receptors (Bergman et al., 2013). Its modest selectivity for dopamine D3 over D2 receptors made it a viable FDA-approved medication to test the dopamine D3 receptor hypothesis of cocaine abuse. Although buspirone reliably decreased cocaine self-administration in rhesus (Bergman et al., 2013; Mello et al., 2013) and cynomolgus monkeys (Czoty & Nader, 2015), a 30 mg dose of buspirone failed to alter the reinforcing effects of cocaine in a human laboratory study (Bolin et al. 2016), and a 60 mg dose (the maximum approved dose) failed to prolong abstinence from cocaine use in a multi-site, randomized, double-blind, placebo-controlled pilot study (Winhusen et al., 2014). Imaging data from non-human primates suggest that a 3-fold larger dose of buspirone would be required to occupy a sufficient proportion (e.g., 80%) of dopamine D3 receptors to achieve a therapeutic effect in humans (Kim et al., 2014).
Because lorcaserin and buspirone differentially target pre-synaptic (lorcaserin; 5-HT2C receptors in the VTA) and post-synaptic (buspirone; dopamine D3 receptors in the NAcc) regulators of dopamine neurotransmission, we hypothesize that fixed-dose combinations of these two FDA-approved drugs will produce a therapeutic effect that is greater than the effect of either drug alone (i.e., a supra-additive interaction). Accordingly, the current study evaluated the effectiveness of lorcaserin and buspirone administered alone, and as binary mixtures (fixed ratios of 3:1, 1:1, and 1:3 relative to the ID50 of each drug), to reduce responding for 0.032 mg/kg/inf cocaine under a progressive ratio (PR) schedule of reinforcement in four rhesus monkeys (2 male and 2 female). Dose addition analyses provide a powerful approach to characterize the nature of the interaction between two drugs that produce the same effect (e.g., Tallarida, 2000; Collins et al., 2016b; Gannon et al., 2018b), and were used in the current study to determine if the effects of the drug mixtures differed from those predicted for an additive interaction between lorcaserin and buspirone.
Methods
Subjects
A total of 4 adult rhesus monkeys were used in these studies, two males (PE and BO), weighing between 8 and 11 kg, and two females (AM and RO), weighing between 8 and 10 kg. All monkeys were individually housed in an environmentally controlled vivarium under a 14h/10h light/dark cycle with free access to water in their home cage. Monkeys were fed primate chow (Harlan Teklad, High Protein Monkey Diet, Madison, WI), fresh fruit, and peanuts daily. Although experimental histories differed among these monkeys, all 4 monkeys had self-administered cocaine daily for the 3-4 years prior to completion of these studies. Information on estrous cycle was not recorded for these studies. All monkeys were maintained, and all experiments were performed, in accordance with the Institutional Animal Care and Use Committee, The University of Texas Health Science Center at San Antonio, and with the Guide for the Care and Use of Laboratory Animals (National Research Council 2011).
Surgical Preparation
Monkeys were surgically prepared with an indwelling venous catheter connected to a subcutaneous (SC) vascular access port located in the mid-scapular region as we have previously described (Collins et al., 2016a; Gerak et al., 2016). Briefly, monkeys were initially anesthetized with 10 mg/kg of ketamine (SC; Fort Dodge Laboratories, Fort Dodge, IA, USA), intubated, and maintained on 2 l/min oxygen and isoflurane anesthesia (Butler Animal Health Supply, Grand Prairie, TX, USA). A polyurethane catheter (SIMS Deltec Inc., St. Paul, MN, USA) was implanted in a vein (e.g., jugular or femoral) and connected to a vascular access port (Access Technologies, Skokie, IL, USA).
Apparatus
During self-administration sessions, monkeys were seated in commercially available chairs (Model R001; Primate Products, Miami, FL, USA) and placed in ventilated, sound-attenuating chambers. The front wall of each chamber contained a response panel consisting of two response levers and two stimulus lights that could be illuminated red or green. Cocaine was delivered intravenously (IV) by a syringe driver (Razel Scientific Instruments Inc., Stamford, CT, USA) located outside the chamber. The syringe driver contained a 30 ml syringe that was connected to the vascular port via an IV extension set (Abbott Laboratories, Stone Mountain, GA, USA) equipped with a 20 ga Huber-point needle (Access Technologies). A computer running Med-PC IV software (Med Associates Inc., St. Albans, VT, USA) controlled experimental events and recorded data.
Cocaine Self-Administration
These four monkeys had a history of self-administering cocaine under FR30:TO 180-sec (Collins et al., 2016a) and PR schedules (Gerak et al., 2016); these monkeys also had a history of acute and repeated intra-gastric treatments with lorcaserin, 90 min before the session (Collins et al., 2016a; Gerak et al., 2016). In the current study, responding for 0.032 mg/kg/inf cocaine was maintained under a PR schedule of reinforcement in which the first ratio was set to 50, and the response requirement increased after each infusion according to the following equation: ratio=[5eˆ(infusion number+11)*0.2)]-5. This resulted in the following series of ratio values: 50, 62, 77, 95, 118, 145, 178, 219, 268, 328, 402, 492, 603, 737, 901. Once monkeys were seated in the operant chamber, a loading infusion was administered to fill the catheter with the appropriate concentration of cocaine. Illumination of the green light above the active lever (counterbalanced across monkeys) signaled the start of the session and subsequent drug availability (i.e., discriminative stimulus). Responding on the active lever was reinforced by infusions of 0.032 mg/kg cocaine delivered in conjunction with a 5-sec presentation of the red light above the active lever (i.e., cocaine-associated stimuli). A 180-sec timeout followed each infusion during which time all stimuli were extinguished and responses were recorded but had no scheduled consequence. Upon completion of the timeout, the green light above the active lever was again illuminated and cocaine was available for responding. Responses on the inactive lever were recorded but had no scheduled consequence. The maximum session duration was 4 hr; however, sessions were terminated if a monkey failed to complete a ratio within 40 min (i.e., 40-min limited hold). To demonstrate that responding was maintained by cocaine infusions, saline was occasionally substituted for cocaine. Although there were slight differences in the amount of responding maintained by the 0.032 mg/kg/inf dose of cocaine among the monkeys (Table 2), this dose was ½-log unit smaller than the dose that maintained peak levels of responding in all monkeys (data not shown), and was selected so that it would be possible to observe either increases or decreases in responding following pretreatments with lorcaserin, buspirone, or fixed dose mixtures of lorcaserin and buspirone.
Table 2.
Individual subject data the ID50 and slope parameters for lorcaserin and buspirone to reduce responding for 0.032 mg/kg/inf cocaine under a PR schedule of reinforcement.
| baseline cocaine | baseline saline | |||||||
|---|---|---|---|---|---|---|---|---|
| infusions | final ratio | infusions | final ratio | lorcaserin (SC) | buspirone (SC) | |||
| females | mean (SEM) | mean (SEM) | mean (SEM) | mean (SEM) | ID50 | slope | ID50 | slope |
| AM | 8.6 (0.2) | 251 (12) | 1.5 (0.5) | 48 (12) | 0.63 | –62.1 | 2.02 | −256.0 |
| RO | 12.3 (0.2) | 538 (23) | 1.8 (0.2) | 60 (4) | 0.31 | −114.3 | 6.34 | −252.6 |
|
| ||||||||
| males | ||||||||
|
| ||||||||
| PE | 7.9 (0.2) | 224 (13) | 1.0 (0.3) | 41 (9) | 1.14 | −103.2 | 0.53 | −182.6 |
| BO | 8.2 (0.2) | 232 (12) | 1.7 (0.2) | 59 (3) | 1.54 | −160.0 | 0.19 | −225.0 |
Daily sessions were preceded by a SC injection of saline 15 min before the start of the session. Once responding met stability criteria (number of infusions differed by no more than 2 across 2 consecutive sessions), monkeys were pretreated 15 min before the start of the session with lorcaserin (0.1, 0.32, 1, 3.2 mg/kg; SC), buspirone (0.032, 0.1, 0.32, 1, 3.2 mg/kg; SC), or a mixture of lorcaserin and buspirone. Doses of lorcaserin and buspirone were selected based on previous studies showing that lorcaserin (intra-gastric; Collins et al., 2016a; Gerak et al., 2016) and buspirone (IM; Bergman et al., 2013) are capable of decreasing cocaine self-administration, and expanded until the range included as at least one dose that produced >20% reduction in drug taking, and at least one dose that produced a <80% decrease in drug taking. Mixtures of lorcaserin and buspirone were evaluated at three fixed ratios (3:1, 1:1, and 1:3 lorcaserin:buspirone) relative to the dose of each drug estimated to reduce self-administration to 50% of baseline for individual subjects (ID50). Details of these mixtures are shown in Table 1. The acute effects of each dose of each drug (or pair of drug doses) were evaluated during single session tests that were separated by at least 2 sessions.
Table 1.
Composition of unique dose pairs of mixtures of lorcaserin and buspirone.
| 3 : 1 lorcaserin : buspirone mg/kg (SEM) : mg/kg (SEM) |
1 : 1 lorcaserin : buspirone mg/kg (SEM) : mg/kg (SEM) |
1 : 3 lorcaserin : buspirone mg/kg (SEM) : mg/kg (SEM) |
|---|---|---|
| 0.21 (0.06) : 0.18 (0.11) | 0.14 (0.04) : 0.36 (0.22) | 0.07 (0.02) : 0.54 (0.34) |
| 0.38 (0.11) : 0.32 (0.20) | 0.25 (0.08) : 0.64 (0.40) | 0.13 (0.04) : 0.96 (0.60) |
| 0.68 (0.20) : 0.57 (0.35) | 0.45 (0.14) : 1.13 (0.71) | 0.23 (0.07) : 1.70 (1.06) |
| 1.21 (0.36) : 1.01 (0.63) | 0.81 (0.24) : 2.02 (1.26) | 0.40 (0.12) : 3.03 (1.89) |
| 2.15 (0.64) : 1.79 (1.12) | 1.43 (0.43) : 3.59 (2.24) | 0.72 (0.21) : 5.38 (3.35) |
Drugs
(−)-Cocaine hydrochloride was provided by the National Institute of Drug Abuse Drug Supply Program. Lorcaserin HCl was purchased from MedChem Express (Princeton, NJ), and buspirone HCl was purchased from Sigma-Aldrich (St. Louis, MO). All drugs were dissolved in physiologic saline. Doses are expressed as the salt in mg per kg body weight.
Statistical Analyses
Data for each drug alone are graphically presented as % baseline infusions and reported as the mean (± 1 SEM) for the group, as well as the means for males and females. Dose-response curves (group level) for lorcaserin and buspirone to alter the self-administration of 0.032 mg/kg/inf cocaine were analyzed by one-way repeated measures ANOVA with post-hoc Dunnet’s tests. Linear regression was also used to obtain estimates of the dose required to reduce self-administration to 50% of baseline (ID50), slope, and Y-intercepts for individual subjects. The range of doses analyzed included one ineffective (≥80% of baseline) and one fully-effective dose (≤20% of baseline).
Analysis of Drug Mixtures
Predicted effect levels were calculated for all drug mixtures based on the concept of dose equivalence and the linear regression functions obtained for each drug in individual monkeys. In order to determine the predicted effect level for a dose pair, the dose of buspirone (B) in each fixed dose pair was converted to an equivalent dose of lorcaserin (L) [Beq(L)] using the following functions from Gannon et al. (2018b)
where SlopeB and SlopeL are the slope parameters and intB and intL are the y-intercepts derived from the linear portion of the dose-response curves for buspirone and lorcaserin, respecitvely. Summing the unit dose of L and Beq(L) allows each dose pair to be expressed in terms of total lorcaserin equivalents (Total DoseeqL) which can then be used to calculate the predicated effect level for an additive interaction using the following function.
Predicted additive dose-response curves for each drug mixture represent the mean (± 1 SEM) of the total lorcaserin equivalents (mg/kg) and the mean (± 1 SEM) predicted effect level (% Baseline Infusions) for each fixed dose pair of each mixture of lorcaserin and buspirone.
Predicted and observed dose-response curves were analyzed by linear regression to obtain estimates of the Total DoseeqL required to reduce self-administration to 50% of baseline (ID50). ID50 values for predicted and observed dose-response curves were used to calculate potency ratios (e.g., ID50observed/ID50predicted) for individual monkeys, with departures from additivity deemed significant if the 95% confidence interval for the group did not include 1.
Results
Under baseline conditions, cocaine maintained stable levels of responding across the experiment, with monkeys receiving an average of 9.3 (± 2.0) infusions per session when 0.032 mg/kg/inf cocaine was available, and an average of 1.6 (± 0.4) infusions when saline was available. As shown in Table 2, females earned slightly more cocaine than males, but these differences were not significant. When administered 15 min before the start of the session, both lorcaserin (F[4,12]=19.2; p<0.001) and buspirone (F[5,15]=4.2; p<0.05) dose-dependently decreased responding for cocaine (Figure 1). Although the effects of lorcaserin and buspirone on cocaine self-administration were qualitatively similar between males and females, lorcaserin appeared to be more potent in females [ID50 = 0.44 (95%CI: 0.22-0.88)] than in males [ID50 = 1.33 (0.99-1.78)], whereas buspirone appeared to be more potent in males [ID50 = 0.32 (0.12-0.87)] than in females [ID50 = 3.58 (1.16-10.99)] (Table 2).
Figure 1.
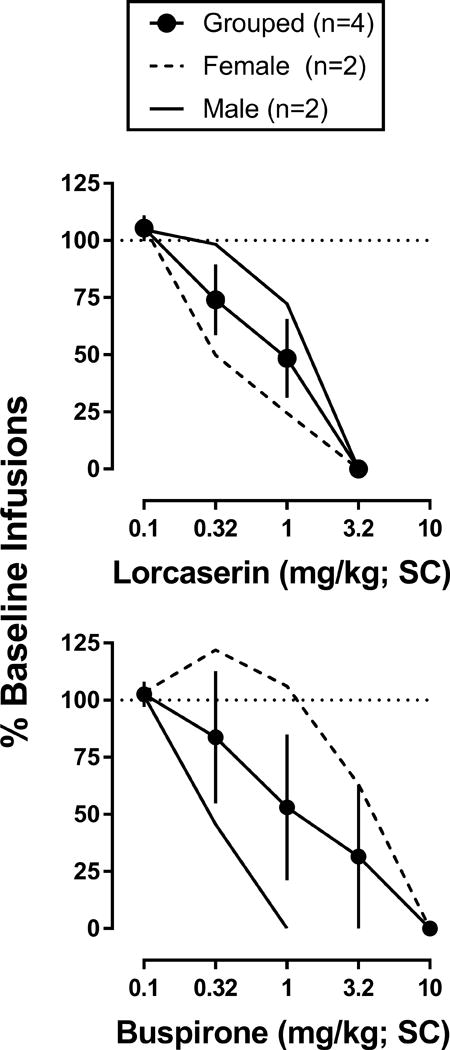
Effects of lorcaserin (circles; top panel) or buspirone (squares; bottom panel) on cocaine infusions (0.032 mg/kg) earned under a PR schedule of reinforcement (n=4; 2 males and 2 females). Mean effects of lorcaserin or buspirone are depicted as a solid line for the 2 male monkeys (PE and BO), and as a dashed line for the 2 female monkeys (AM, and RO). Abscissa: dose of lorcaserin (0.1-3.2 mg/kg) or buspirone (0.1-10 mg/kg) administered SC 15 min before the start of the session. Ordinate: percent of baseline infusions expressed as the mean (± 1 SEM) for the number of infusions earned following pretreatment with lorcaserin or buspirone relative to the average number of infusions earned during the two preceding baseline sessions. *p<0.05; **p<0.01; ***p<0.001. Significant effects of lorcaserin or buspirone on responding for cocaine were determined by one-way, repeated measures ANOVA with post-hoc Dunnett’s tests.
As shown in Figure 2, when administered at fixed ratios of 3:1, 1:1, and 1:3 relative to their ID50s from individual subjects, mixtures of lorcaserin and buspirone dose-dependently decreased cocaine self-administration (3:1 – F[5,15]=6.9; p<0.01; 1:1 – F[5,15]=11.9; p<0.001; 1:3 – F[5,15]=5.4; p<0.01). Although 10 of the 12 individual ID50 values for mixtures of lorcaserin and buspirone were smaller than predicted for an additive interaction, a statistically significant supra-additive interaction (potency ratio less than 1) was observed only when lorcaserin and buspirone were mixed at a 1:1 ratio of their ID50s (Figure 3). Although interactions between lorcaserin and buspirone were found to be additive when mixed at 3:1 or 1:3 ratios of their ID50s, the 1:3 mixture of lorcaserin and buspirone showed large departures from additivity in the supra-additive direction for 3 of the 4 monkeys, with the fourth monkey exhibiting a sub-additive interaction. A similar trend was observed for the 3:1 mixture of lorcaserin and buspirone; however, the magnitude of the supra-additive interactions tended to be smaller than for the other mixtures. There were no apparent sex-differences in the effects of mixtures of lorcaserin and buspirone. In all cases, responding for cocaine returned to baseline levels by 24 hr after the administration of lorcaserin, buspirone, or lorcaserin and buspirone mixtures (data not shown).
Figure 2.
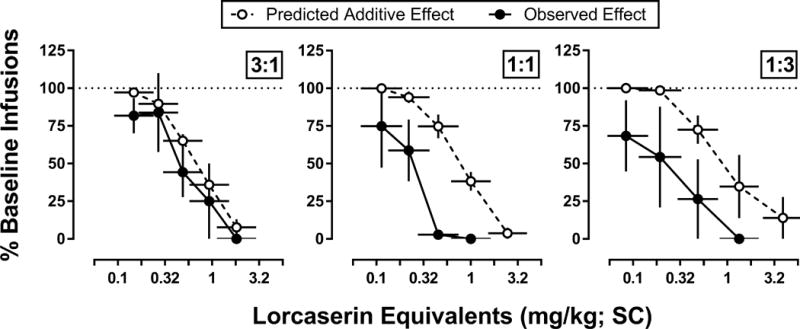
Predicted (open circles; dashed line) and observed (circles; solid line) effects of mixtures of lorcaserin and buspirone on cocaine infusions (0.032 mg/kg) earned under a PR schedule of reinforcement (n=4; 2 males and 2 females). Mixtures of lorcaserin and buspirone were evaluated at three fixed dose ratios (3:1, 1:1, and 1:3) relative to the ID50 value for each drug from individual subjects. Abscissa: mean (± 1 SEM) of the total mixture dose, expressed in mg/kg of lorcaserin equivalents, administered SC 15 min before the start of the session. Ordinate: percent of baseline infusions expressed as the mean (± 1 SEM) for the number of infusions earned following pretreatment with mixtures of lorcaserin and buspirone relative to the average number of infusions earned during the two preceding baseline sessions.
Figure 3.
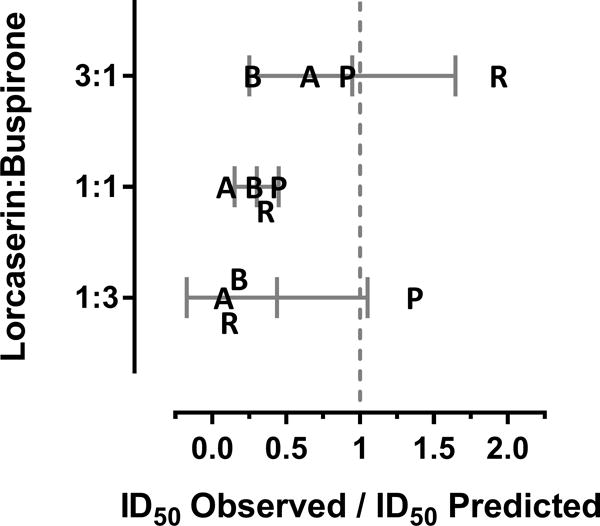
Potency ratios (ID50 observed/ID50 predicted additive) for binary mixtures of lorcaserin and buspirone at three different fixed dose ratios (3:1, 1:1, and 1:3). Abscissa: mean potency ratios (± 95% confidence intervals) are depicted by the vertical gray lines and error bars; potency ratios determined for individual subjects identified by the first letter of their ID (B and P denote males; A and R denote females). Ordinate: identifies the ratio at which lorcaserin and buspirone were mixed. Potency ratios for which the 95% confidence intervals do not include 1 indicate that the interaction departs from additivity, with ratios less than 1 indicative of a supra-additive interaction, and ratios greater than 1 indicative of a sub-additive interaction.
Discussion
Preclinical evidence suggests that buspirone and lorcaserin can decrease cocaine self-administration following both acute and repeated (sub-chronic) administration, making them attractive candidates for the treatment of cocaine use disorder. The results of the current studies confirm these preclinical findings, and extend them by examining whether fixed-dose combinations of lorcaserin (a 5-HT2C receptor-preferring agonist) and buspirone (a 5-HT1A receptor partial agonists and dopamine D3/D4 receptor antagonist) would be more potent and/or effective at reducing the reinforcing effectiveness of cocaine than predicted for an additive interaction. There were three main findings of this study, as follows: 1) when administered alone, lorcaserin and buspirone each dose-dependently decreased cocaine self-administration; 2) when administered as 1:1 or 1:3 ratios relative to their ID50s, mixtures of lorcaserin and buspirone were 4-7 fold more potent at reducing responding for cocaine than predicted for an additive interaction; and 3) the nature of the interaction between lorcaserin and buspirone varied as a function of the ratio at which they were mixed, with a supra-additive interaction observed at the 1:1 ratio and additive interactions observed for both the 1:3 and 3:1 mixtures of lorcaserin and buspirone. Together, these findings provide support for the hypothesis and suggest that combining these two FDA-approved drugs could provide a novel, effective, and highly translatable pharmacotherapy for treating cocaine use disorder.
Consistent with previous studies in rodents and non-human primates, both lorcaserin (Harvey-Lewis et al., 2016; Collins et al., 2016a; Gerak et al., 2016; Gannon et al., 2018a) and buspirone (Gold & Balster, 1992; Homberg et al., 2004; Bergman et al., 2013; Mello et al., 2013) decreased the self-administration of 0.032 mg/kg/inf cocaine under a PR schedule of reinforcement. In the current study, buspirone appeared to be 10-fold more potent at decreasing cocaine self-administration in males than in females, whereas lorcaserin appeared to be slightly (~3-fold) more potent in females than in males; however, it is important to note that these studies were not designed (i.e., powered) to detect sex-related differences. Nevertheless, clinical data support this apparent sex-related difference, with a 60 mg dose of buspirone being more effective at promoting abstinence from cocaine in males than in females (Winhusen et al., 2014). Although these findings provide additional support for the development of 5-HT2C receptor agonists as well as drugs with antagonist actions at dopamine D2-like (D2, D3, and D4) receptors as pharmacotherapies for cocaine abuse, translating preclinical success to effective therapies in the clinic has been challenging. In a recent review describing progress in the preclinical development and clinical evaluation of medications for cocaine abuse, Czoty, Stoops, and Rush (2016) discuss some of the major hurdles, including differences in study endpoints (i.e., reductions in preclinical drug-taking versus complete abstinence in the clinic), as well as the fact that clinical evaluations of candidate medications are limited to the doses approved by the FDA for treating the primary indication of a drug (e.g., 10 mg BID for lorcaserin, and 30 mg BID for buspirone).
Indeed, despite consistent decreases in cocaine-taking in preclinical studies (e.g., Bergman et al., 2013; Mello et al., 2013), a small dose of buspirone (30 mg) failed to alter the reinforcing effects of cocaine in a human laboratory study (Bolin et al., 2016), and the maximal approved dose of buspirone (60 mg) failed to prolong abstinence from cocaine use in a multi-site, randomized, double-blind, placebo-controlled pilot study (Winhusen et al., 2014). Although these results largely halted efforts to develop buspirone as a pharmacotherapy for cocaine abuse, it is important to note that imaging data from non-human primates suggest that a clinically relevant level of dopamine D3 receptor occupancy in humans would require buspirone to be administered at doses at least 3-fold larger than the 60 mg maximal dose approved for treating anxiety (Kim et al., 2014). Although it is too early to tell whether the maximal approved dose of lorcaserin (10 mg BID) will be an effective pharmacotherapy for cocaine use disorder (there are currently 7 trails registered with clinicaltrials.gov), doses only slightly larger than those approved to treat obesity have been reported to produce feelings of “high”, “bad effects”, and “hallucination” in polydrug users (Shram et al., 2011). These effects are consistent with lorcaserin having agonist actions at 5-HT2A receptors, a notion that is supported by a recent study by Serafine et al. (2015) in which lorcaserin induced 5-HT2A receptor-mediated head-twitches in rats at doses comparable to those required to reduce body weight in rats (Higgins & Fletcher, 2015). While it is difficult to make direct comparisons of dose (and their corresponding blood levels) across species, the blood levels of lorcaserin (~390 nmol/L) associated with a 50% decrease in PR responding for 0.032 mg/kg/inf cocaine in rhesus monkeys (Collins et al., 2016a; Gerak et al., 2016) are ~3-times greater than those observed in humans treated with the maximally approved dose of lorcaserin (~131 nmol/L; Smith et al., 2009). In addition to suggesting that that the 10 mg (BID) dose of lorcaserin might not be sufficient when evaluated in a clinical population, these findings also suggest that the doses of lorcaserin required to decrease responding for cocaine in rhesus monkeys might have actions at both 5-HT2C and 5-HT2A receptors (for review see, Collins et al., 2017). Thus, in addition to developing novel therapeutics, there is a clear need to develop strategies to enhance/augment the potency and/or effectiveness of FDA-approved drugs so that abstinence from cocaine use can be achieved with doses that are already approved to treat other conditions.
The current studies sought to improve/increase the potency and/or effectiveness of lorcaserin and buspirone to reduce cocaine self-admiration by co-administering these two drugs as a mixture. Because the nature of drug-drug interactions (e.g., additive, sub-additive, supra-additive) can vary depending upon the ratio at which drugs are combined, lorcaserin and buspirone were evaluated as mixtures containing 3:1, 1:1, and 1:3 ratios of lorcaserin:buspirone relative their ID50 values from individual subjects. Although the effects of the 3:1 mixture of lorcaserin and buspirone were additive in nature (i.e., the observe and predicted dose-response curves did not differ), the 1:1 and 1:3 mixtures were more potent than predicted for an additive interaction, as evidenced by their dose-response curves falling to the left of the predicted additive dose-response curve. Indeed, the 1:1 mixture was at least 2-fold more potent than predicted in all 4 monkeys, with a 9-fold increase in potency observed for monkey AM. Although the magnitude of this interaction appeared to be greater when lorcaserin and buspirone were mixed at a 3:1 ratio, the interaction was sub-additive in one monkey (PE), suggesting that drug-drug interactions can also vary across subjects. Translating these effects into changes in absolute doses of each constituent drug, the 1:1 mixture resulted in ~7-fold (±1.6) reductions in the constituent ID50s (i.e., the amount of each constituent drug comprising the ID50 for the mixture) for both lorcaserin and buspirone, whereas the 1:3 mixture resulted in a ~25-fold (±8.9) reduction the constituent ID50 for lorcaserin, and a ~8-fold (±3.0) reduction in the constituent ID50 for buspirone to reduce cocaine self-administration. Supra-additive interactions translate to larger reductions in potency for constituent drugs; however, even the 3:1 mixture, which exhibited strictly additive interactions, reduced the constituent ID50s by ~2-fold (±1.0) for lorcaserin and ~8-fold (±3.9) for buspirone. Although a combination therapy containing mixtures of lorcaserin and buspirone does not directly address issues thought to underlie the low clinical effectiveness of buspirone (e.g., too few receptors occupied; Kim et al., 2014), the fact that reductions in cocaine self-administration were achieved with smaller doses of lorcaserin and buspirone when these drugs were administered as mixtures suggests that the high receptor occupancy thought to be necessary for a therapeutic effect with a single drug (e.g., buspirone) might not be necessary when drugs with complimentary mechanisms of action are combined.
Taken together, the results of these studies provide support for the development of combination therapies targeting 5-HT2C receptor agonists, such as lorcaserin, and dopamine D3 (and/or D4) receptor antagonists, such as buspirone, for treating cocaine use disorder. There are, however, several limitations of the current studies, including the following: 1) they characterized only the acute interactions between lorcaserin and buspirone; 2) they evaluated the effectiveness of lorcaserin, buspirone, and mixtures of lorcaserin and buspirone to alter the reinforcing effects of a single dose of cocaine; 3) they included a relatively small sample size and, therefore, were unable to determine whether apparent sex-related differences in the effects of lorcaserin and buspirone were statistically significant; 4) they focused on interactions between lorcaserin and buspirone with respect to their effectiveness to alter cocaine self-administration, without also evaluating interactions for other, potentially adverse, effects (e.g., hallucination); and 5) they did not rule out a potential contribution of buspirone’s partial agonist actions at 5-HT1A receptors to its effectiveness to decrease cocaine self-administration, or its interactions with lorcaserin. Accordingly, future studies will need to extend these initial findings to evaluate the effects of repeated/chronic administration of mixtures of lorcaserin and buspirone against a wider range of cocaine doses, and in larger groups of male and female monkeys. Moreover, although preclinical evidence suggests that the therapeutic effects of lorcaserin extend to other drugs of abuse, including alcohol, nicotine, opioids, and other stimulants (Higgins et al., 2012; Rezvani et al., 2014; Gerak et al., 2016; Neelakantan et al., 2017; Gannon et al., 2018a), further studies will be required to determine whether mixtures of lorcaserin and buspirone produce a similar additive/supra-additive inhibition of self-administration maintained by drugs other than cocaine.
Public Health Statement.
Cocaine use disorder remains a serious public health problem, for which there are no FDA-approved pharmacotherapies. These studies show that administering two FDA-approved drugs that target presynaptic (5-HT2C receptors; lorcaserin, approved to treat obesity) and postsynaptic (dopamine D3 receptors; buspirone, approved to treat anxiety) regulators of dopamine neurotransmission results in a supra-additive (i.e., synergistic) reduction in cocaine self-administration, and could provide a highly translatable approach to treating cocaine use disorder in humans.
Abbreviations
- 5-HT
serotonin
- DAT
dopamine transporter
- FDA
Food and Drug Administration
- ID50
inhibitory dose 50%
- IV
intravenous
- NAcc
nucleus accumbens
- PR
progressive ratio
- SC
subcutaneous
- VTA
ventral tegmental area
Contributor Information
Gregory T. Collins, Department of Pharmacology, University of Texas Health Science Center at San Antonio, 7703 Floyd Curl Dr., San Antonio, TX, 78229, USA Addiction Research, Treatment & Training Center of Excellence, University of Texas Health Science Center at San Antonio, 7703 Floyd Curl Dr., San Antonio, TX, 78229, USA; South Texas Veterans Health Care System, 7400 Merton Minter Dr., San Antonio, TX, 78229, USA.
Charles P. France, Department of Pharmacology, University of Texas Health Science Center at San Antonio, 7703 Floyd Curl Dr., San Antonio, TX, 78229, USA Addiction Research, Treatment & Training Center of Excellence, University of Texas Health Science Center at San Antonio, 7703 Floyd Curl Dr., San Antonio, TX, 78229, USA.
References
- Alex KD, Pehek EA. Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol Ther. 2007;113:296–320. doi: 10.1016/j.pharmthera.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Negus SS. Repeated 7-Day treatment with the 5-HT2C agonist lorcaserin or the 5-HT2A antagonist pimavanserin alone or in combination fails to reduce cocaine vs food choice in male rhesus monkeys. Neuropsychopharmacology. 2016;42:1082–1092. doi: 10.1038/npp.2016.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman J, Roof RA, Furman CA, Conroy JL, Mello NK, Sibley DR, Skolnick P. Modification of cocaine self-administration by buspirone (Buspar®): potential involvement of D3 and D4 dopamine receptors. Int J Neuropsychopharmacol. 2013;16:445–458. doi: 10.1017/S1461145712000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolin BL, Lile JA, Marks KR, Beckmann JS, Rush CR, Stoops WW. Buspirone reduces sexual risk-taking intent but not cocaine self-administration. Exp Clin Psychopharmacol. 2016;24:162–173. doi: 10.1037/pha0000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubar MJ, Cunningham KA. Distribution of serotonin 5-HT2C receptors in the ventral tegmental area. Neuroscience. 2007;146:286–297. doi: 10.1016/j.neuroscience.2006.12.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Gerak LR, Javors MA, France CP. Lorcaserin reduces the discriminative stimulus and reinforcing effects of cocaine in rhesus monkeys. J Pharmacol Exp Ther. 2016a;356:85–95. doi: 10.1124/jpet.115.228833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Abbott M, Galindo K, Rush EL, Rice KC, France CP. Discriminative stimulus effects of binary drug mixtures: studies with cocaine, MDPV, and caffeine. J Pharmacol Exp Ther. 2016b;359:1–10. doi: 10.1124/jpet.116.234252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Gerak LR, France CP. The behavioral pharmacology and therapeutic potential of lorcaserin for treating substance use disorders. Neuropharmacology. 2017 doi: 10.1016/j.neuropharm.2017.12.023. (e-pub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KA, Fox RG, Anastasio NC, Bubar MJ, Stutz SJ, Moeller FG, Gilbertson SR, Rosenzweig-Lipson S. Selective serotonin 5-HT(2C) receptor activation suppresses the reinforcing efficacy of cocaine and sucrose but differentially affects the incentive-salience value of cocaine- vs. sucrose-associated cues. Neuropharmacology. 2011;61:513–523. doi: 10.1016/j.neuropharm.2011.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Nader MA. Effects of oral and intravenous administration of buspirone on food-cocaine choice in socially housed male cynomolgus monkeys. Neuropsychopharmacology. 2015;40:1072–1083. doi: 10.1038/npp.2014.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Stoops WW, Rush CR. Evaluation of the pipeline for development of medications for cocaine use disorder: a review of translational preclinical, human laboratory, and clinical trial research. Pharmacol Rev. 2016;68:533–562. doi: 10.1124/pr.115.011668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacki SC, O’Brien C. Glutamatergic agents for cocaine dependence. Ann N Y Acad Sci. 2003;1003:328–345. doi: 10.1196/annals.1300.021. [DOI] [PubMed] [Google Scholar]
- DiMasi JA, Grabowski HG, Hansen RW. Innovation in the pharmaceutical industry: New estimates of R&D costs. J Health Econ. 2016;47:20–33. doi: 10.1016/j.jhealeco.2016.01.012. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Chintoh AF, Sinyard J, Higgins GA. Injection of the 5-HT2C receptor agonist Ro60-0175 into the ventral tegmental area reduces cocaine-induced locomotor activity and cocaine self-administration. Neuropsychopharmacology. 2004;29:308–318. doi: 10.1038/sj.npp.1300319. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Rizos Z, Sinyard J, Tampakeras M, Higgins GA. The 5-HT2C receptor agonist Ro60-0175 reduces cocaine self-administration and reinstatement induced by the stressor yohimbine, and contextual cues. Neuropsychopharmacology. 2008;33:1402–1412. doi: 10.1038/sj.npp.1301509. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Sinyard J, Higgins GA. Genetic and pharmacological evidence that 5-HT2C receptor activation, but not inhibition, affects motivation to feed under a progressive ratio schedule of reinforcement. Pharmacol Biochem Behav. 2010;97:170–178. doi: 10.1016/j.pbb.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Gannon BM, Galindo KI, Mesmin MP, Sulima A, Rice KC, Collins GT. Relative reinforcing effects of second-generation synthetic cathinones: acquisition of self-administration and fixed ratio dose-response curves in rats. Neuropharmacology. 2017 doi: 10.1016/j.neuropharm.2017.08.018. (e-pub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Sulima A, Rice KC, Collins GT. Inhibition of cocaine and 3,4-methylenedioxypyrovalerone (MDPV) self-administration by lorcaserin is mediated by 5-HT2C receptors in rats. J Pharmacol Exp Ther. 2018a;364:359–366. doi: 10.1124/jpet.117.246082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Galindo KI, Mesmin MP, Rice KC, Collins GT. Reinforcing effects of binary mixtures of common bath salts constituents: studies with 3,4-methylenedioxypyrovalerone (MDPV), 3,4- methylenedioxymethcathinone (methylone), and caffeine in rats. Neuropsychopharmacology. 2018b;43:761–769. doi: 10.1038/npp.2017.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerak LR, Collins GT, France CP. Effects of lorcaserin on cocaine and methamphetamine self-administration and reinstatement of responding previously maintained by cocaine in rhesus monkeys. J Pharmacol Exp Ther. 2016;359:383–391. doi: 10.1124/jpet.116.236307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold LH, Balster RL. Effects of buspirone and gepirone on i.v. cocaine self-administration in rhesus monkeys. Psychopharmacology (Berl) 1992;108:289–94. doi: 10.1007/BF02245114. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Shearer J, Merrill J, Negus SS. Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict Behav. 2004;29:1439–1464. doi: 10.1016/j.addbeh.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Grottick AJ, Fletcher PJ, Higgins GA. Studies to investigate the role of 5-HT(2C) receptors on cocaine- and food-maintained behavior. J Pharmacol Exp Ther. 2000;295:1183–1191. [PubMed] [Google Scholar]
- Gurevich EV, Joyce JN. Distribution of dopamine D3 receptor expressing neurons in the human forebrain: comparison with D2 receptor expressing neurons. Neuropsychopharmacology. 1999;20:60–80. doi: 10.1016/S0893-133X(98)00066-9. [DOI] [PubMed] [Google Scholar]
- Harvey-Lewis C, Li Z, Higgins GA, Fletcher PJ. The 5-HT(2C) receptor agonist lorcaserin reduces cocaine self-administration, reinstatement of cocaine-seeking and cocaine induced locomotor activity. Neuropharmacology. 2016;101:237–245. doi: 10.1016/j.neuropharm.2015.09.028. [DOI] [PubMed] [Google Scholar]
- Heidbreder C. Selective antagonism at dopamine D3 receptors as a target for drug addiction pharmacotherapy: a review of preclinical evidence. CNS Neurol Disord Drug Targets. 2008;7:410–421. doi: 10.2174/187152708786927822. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Silenieks LB, Rossmann A, Rizos Z, Noble K, Soko AD, Fletcher PJ. The 5-HT2C receptor agonist lorcaserin reduces nicotine self-administration, discrimination, and reinstatement: relationship to feeding behavior and impulse control. Neuropsychopharmacology. 2012;37:1177–1191. doi: 10.1038/npp.2011.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins GA, Fletcher PJ. Therapeutic potential of 5-HT2C receptor agonists for addictive disorders. ACS Chem NeuroSci. 2015;6:1071–1088. doi: 10.1021/acschemneuro.5b00025. [DOI] [PubMed] [Google Scholar]
- Homberg JR, Arends B, Wardeh G, Raasø HS, Schoffelmeer AN, de Vries TJ. Individual differences in the effects of serotonergic anxiolytic drugs on the motivation to self-administer cocaine. Neuroscience. 2004;128:121–130. doi: 10.1016/j.neuroscience.2004.05.048. [DOI] [PubMed] [Google Scholar]
- Howell LL, Cunningham KA. Serotonin 5-HT2 receptor interactions with dopamine function: implications for therapeutics in cocaine use disorder. Pharmacol Rev. 2015;67:176–197. doi: 10.1124/pr.114.009514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SW, Fowler JS, Skolnick P, Muench L, Kang Y, Shea C, Logan J, Kim D, Carter P, King P, Alexoff D, Volkow ND. Therapeutic doses of buspirone block D3 receptors in the living primate brain. Int J Neuropsychopharmacology. 2014;17:1257–1267. doi: 10.1017/S1461145714000194. [DOI] [PubMed] [Google Scholar]
- Lévesque D, Diaz J, Pilon C, Martres MP, Giros B, Souil E, Schott D, Morgat JL, Schwartz JC, Sokoloff P. Identification, characterization, and localization of the dopamine D3 receptor in rat brain using 7-[3H]hydroxy-N,N-di-n-propyl-2-aminotetralin. Proc Natl Acad Sci. 1992;89:8155–8159. doi: 10.1073/pnas.89.17.8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK. Preclinical evaluation of the effects of buprenorphine, naltrexone and desipramine on cocaine self-administration. NIDA Res Monograph. 1990;105:189–195. [PubMed] [Google Scholar]
- Mello NK, Fivel PA, Kohut SJ, Bergman J. Effects of chronic buspirone treatment on cocaine self-administration. Neuropsychopharmacology. 2013;38:455–467. doi: 10.1038/npp.2012.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. 8th. Washington, DC: National Academies Press; 2011. [Google Scholar]
- Navailles S, Moison D, Cunningham KA, Spampinato U. Differential regulation of the mesoaccumbens dopamine circuit by serotonin2C receptors in the ventral tegmental area and the nucleus accumbens: an in vivo microdialysis study with cocaine. Neuropsychopharmacology. 2008;33:237–246. doi: 10.1038/sj.npp.1301414. [DOI] [PubMed] [Google Scholar]
- Neelakantan H, Holliday ED, Fox RG, Stutz SJ, Comer SD, Haney M, Anastasio NC, Moeller FG, Cunningham KA. Lorcaserin suppresses oxycodone self-Administration and relapse vulnerability in rats. ACS Chem Neurosci. 2017;8:1065–1073. doi: 10.1021/acschemneuro.6b00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Henningfield J. Agonist medications for the treatment of cocaine use disorder. Neuropsychopharmacology. 2015;40:1815–1825. doi: 10.1038/npp.2014.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AH, Grundt P, Nader MA. Dopamine D3 receptor partial agonists and antagonists as potential drug abuse therapeutic agents. J Med Chem. 2005;48:3663–3679. doi: 10.1021/jm040190e. [DOI] [PubMed] [Google Scholar]
- Newman AH, Blaylock BL, Nader MA, Bergman J, Sibley DR, Skolnick P. Medication discovery for addiction: translating the dopamine D3 receptor hypothesis. Biochem Pharmacol. 2012;84:882–890. doi: 10.1016/j.bcp.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt DM, Rowlett JK, Spealman RD. Behavioral effects of cocaine and dopaminergic strategies for preclinical medication development. Psychopharmacology (Berl) 2002;163:265–282. doi: 10.1007/s00213-002-1137-8. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Cauley MC, Levin ED. Lorcaserin, a selective 5-HT(2C) receptor agonist, decreases alcohol intake in female alcohol preferring rats. Pharmacol Biochem Behav. 2014;125:8–14. doi: 10.1016/j.pbb.2014.07.017. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Brebner K. GABA modulation of cocaine self-administration. Ann N Y Acad Sci. 2000;909:145–158. doi: 10.1111/j.1749-6632.2000.tb06680.x. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Prisinzano TE, Newman AH. Dopamine transport inhibitors based on GBR12909 and benztropine as potential medications to treat cocaine addiction. Biochem Pharmacol. 2008;75:2–16. doi: 10.1016/j.bcp.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafine KM, Rice KC, France CP. Directly observable behavioral effects of lorcaserin in rats. J Pharmacol Exp Ther. 2015;355:381–385. doi: 10.1124/jpet.115.228148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shram MJ, Schoedel KA, Bartlett C, Shazer RL, Anderson CM, Sellers EM. Evaluation of the abuse potential of lorcaserin, a serotonin 2C (5-HT2C) receptor agonist, in recreational polydrug users. Clin Pharmacol Ther. 2011;89:683–692. doi: 10.1038/clpt.2011.20. [DOI] [PubMed] [Google Scholar]
- Smith SR, Prosser WA, Donahue DJ, Morgan ME, Anderson CM, Shanahan WR, APD356-004 Study Group Lorcaserin (APD356), a selective 5-HT(2C) agonist, reduces body weight in obese men and women. Obesity (Silver Spring) 2009;17:494–503. doi: 10.1038/oby.2008.537. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ. In: The composite additive curve, in Drug synergism and dose-effect data analysis. Tallarida RJ, editor. CRC Press; Boca Raton, FL: 2000. pp. 77–89. [Google Scholar]
- Tecott LH, Sun LM, Akana SF, Strack AM, Lowenstein DH, Dallman MF, Julius D. Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature. 1995;374:542–546. doi: 10.1038/374542a0. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Hall FS, Uhl GR, Caine SB. Dramatically decreased cocaine self-administration in dopamine but not serotonin transporter knock-out mice. J Neurosci. 2009;29:1087–1092. doi: 10.1523/JNEUROSCI.4037-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime, World Drug Report 2016 (United Nations publication, Sales No. E.16.XI.7)
- Vickers SP, Clifton PG, Dourish CT, Tecott LH. Reduced satiating effect of d-fenfluramine in serotonin 5-HT(2C) receptor mutant mice. Psychopharmacology (Berl) 1999;143:309–314. doi: 10.1007/s002130050952. [DOI] [PubMed] [Google Scholar]
- Winhusen TM, Kropp F, Lindblad R, Douaihy A, Haynes L, Hodgkins C, Chartier K, Kampman KM, Sharma G, Lewis DF, VanVeldhuisen P, Theobald J, May J, Brigham GS. Multisite, randomized, double-blind, placebo-controlled pilot clinical trial to evaluate the efficacy of buspirone as a relapse-prevention treatment for cocaine dependence. J Clin Psychiatry. 2014;75:757–764. doi: 10.4088/JCP.13m08862. [DOI] [PMC free article] [PubMed] [Google Scholar]


