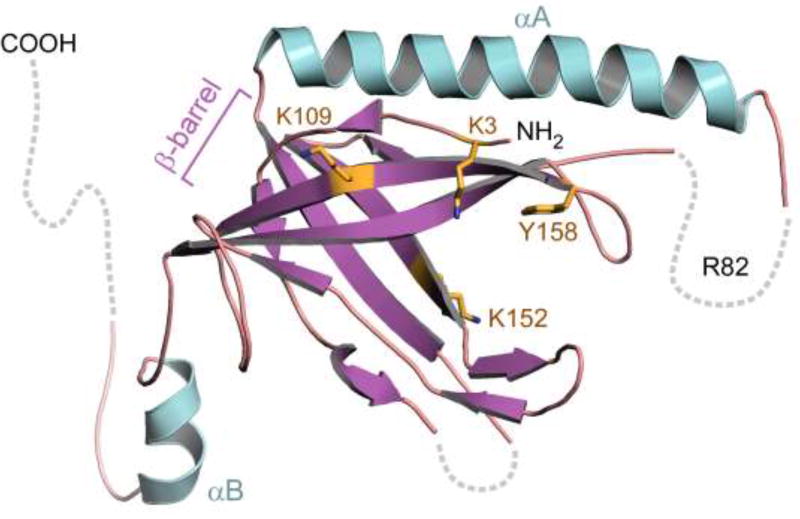
Figure 2.
Crystal Structure of T7 gp2.5. Gp2.5 consists of a single OB-fold domain containing an anti-parallel five-stranded β-barrel (purple). Basic and aromatic acid residues implicated in DNA binding (K3, K109, K152, Y158) lie in the β-barrel and are indicated yellow. Alpha helix A (αA, turquoise) is located on the opposite face of the β-barrel relative to other prokaryotic ssDNA binding proteins. R84, a residue critical for the ability of gp2.5 to mediate homologous ssDNA annealing, is located in a disordered loop connecting αA to β3 (dashed line). The C-terminal tail, not present in the construct used for crystallization, is likely disordered and is shown protruding away from the β-barrel (dashed line).