Fig. 1.
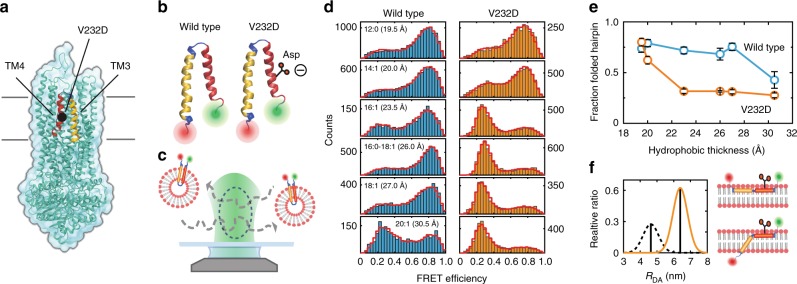
CFTR wild-type and V232D mutant TM3/4 hairpin folding probed by single-molecule FRET. a Structure of CFTR3 highlighting the position of the V232D mutation in TM3/4 (yellow/red). b Schematic representation of the wild-type (left) and V232D (right) TM3/4 helical-hairpin motifs comprising CFTR’s transmembrane helices TM3 (yellow) and TM4 (red). c Schematic of the single-molecule FRET approach for investigating hairpin conformations. Shown are single fluorescently labeled TM3/4 hairpin molecules reconstituted into phospholipid vesicles freely diffusing through the observation volume of the confocal microscope. d FRET efficiency histograms of wild-type (blue) and V232D TM3/4 (orange) in PC lipid vesicles with 12:0, 14:1, 16:1, 16:0–18:1 (POPC), 18:1, and 20:1 acyl chains. Distances between the acyl chain C-2 atoms are indicated as measures of hydrophobic thicknesses48. PDA fits to the histograms are shown as red cityscapes. e Fraction of folded hairpin as function of hydrophobic thickness for wild-type TM3/4 (blue) and V232D TM3/4 (orange) as determined by PDA fits. Errors are standard deviations of the PDA chi-square minimization algorithm calculated from ten iterations. f Closed-state (black dashed) and open-state (orange solid) interfluorophore distance (RDA) distributions for V232D TM3/4 in POPC determined using PDA (left panel), in accordance with a fully extended interfacially bound hairpin or a partially inserted hairpin with TM3 being inserted and TM4 positioned atop the bilayer (right panels)