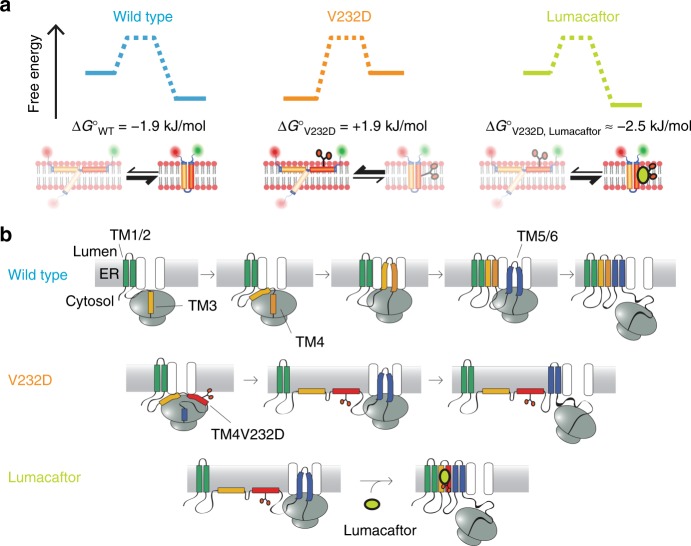
Fig. 3.
TM3/4 hairpin equilibria and mechanistic models for V232D-induced misfolding and drug rescue by Lumacaftor. a TM3/4 hairpin equilibria of wild type, V232D, and V232D upon rescue with Lumacaftor. ΔG° values are given for POPC bilayers. b Upper row: Model of wild-type CFTR topogenesis at the endoplasmic reticulum, adapted from Kim and Skach14. Transmembrane segments are integrated in a pairwise manner into the endoplasmic reticulum membrane. After integration of TM1/2, TM3 and TM4 simultaneously insert as a helical hairpin as TM3 encodes an inefficient signal sequence and thus cooperates with TM4 to translocate the intervening extracellular loop into the membrane. Middle row: Topogenesis model for misfolding of the V232D mutant. For clarity, one example of V232D TM3/4 positioning is depicted here, with both helices interfacially bound, with the alternative being a partially inserted state for TM3 (see a and Fig. 1f). The latter is not shown as it represents a highly unlikely situation that would necessitate an inverted topology of TM3/4 with the hydrophilic intervening loop between TM3 and TM4 spanning the hydrophobic core region of the membrane. Lower row: Model of reversal of V232D misfolding by small-molecule corrector Lumacaftor (see Discussion for potential mechanisms)