Fig. 2.
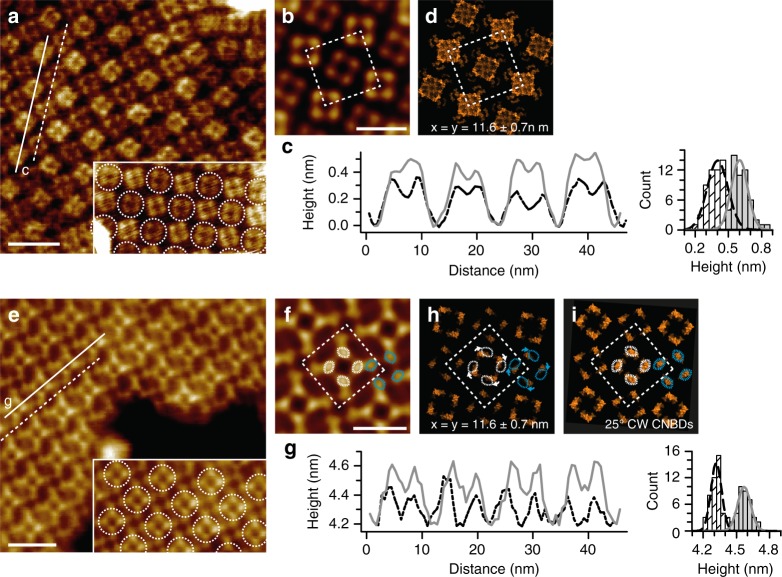
Characterization of the SthK 2D-crystals in presence of cAMP. a HS-AFM image of a 2D-crystal viewed from the extracellular side. Each channel appears as a square-shaped tetramer. Each protruding turret of the pore domain within the channel tetramer is clearly resolved. Inset: The alternating packing of the channels is highlighted with dashed outlines (scale bar: 15 nm). b Four-fold symmetrized correlation average of a. The unit cell (dashed square, dimensions a = b = 11.6 nm, γ = 90°) comprises two tetramers (scale bar: 10 nm). c Left: Height profiles of SthK molecules along the dashed and solid lines in a are shown by the black and grey traces, respectively. Right: Height histogram of the two classes of molecules with 0.3 and 0.6 nm average protrusion heights. d Packing model of the 2D-crystal (PDB 6CJQ). e, f, g, h same as a, b, c, and d, but the 2D-crystal is imaged from the intracellular side exposing the CNBDs. Dashed circles in a and e highlight the alternating packing of the tetramers in the 2D-crystal. The stronger-protruding CNBDs on the intracellular side (e, f) are the less protruding ones from the extracellular side (a, b). White arrows in h indicate the CW rotation that the CNBDs should undergo upon cAMP-binding to match the HS-AFM data. The dotted white and cyan circles in h show the position of the protrusions in the data, which do not match the packing of the resting state SthK model. i Structural model with 25° CW rotated CNBDs matches the experimental data (compare with h). The white and cyan dashed circles in f highlight the two neighboring tetramers of high and low height, respectively. The 25° CW rotation of the CNBDs in the model brings the model CNBD back into the white and cyan dashed circles (i)