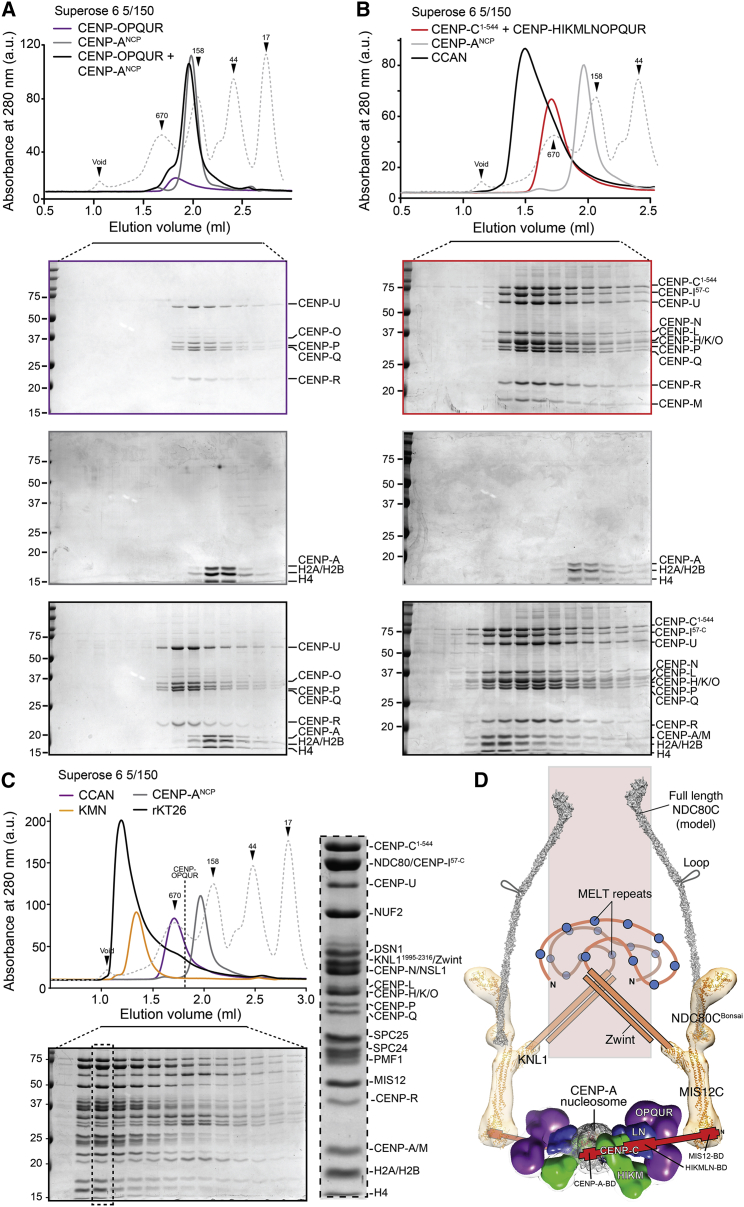
Figure 4.
Reconstitution of a 26-Subunit Kinetochore (rKT26)
(A) Elution profile from analytical SEC, and subsequent SDS-PAGE analysis, of a mixture of CENP-OPQUR (5 μM) complex and CENP-ANCPs (2.5 μM).
(B) Elution profile from analytical SEC, and subsequent SDS-PAGE analysis, of a stoichiometric mixture of CENP-C1–544, CENP-HIΔ56KMLN-OPQUR (each at 5 μM), and CENP-ANCPs (2.5 μM).
(C) Elution profile from analytical SEC, and subsequent SDS-PAGE analysis, of a mixture of CENP-C1-544 (red trace), the CCAN core (CENP-HIΔ56KMLN-OPQUR, violet trace), the KNM network (orange trace) (each at 5 μM), and the CENP-A nucleosomes (grey trace) (2.5 μM) resulting in the formation of a 26-subunit complex (black trace) that links the centromeric DNA to microtubules. Enlargement of the dotted lane of the SDS-PAGE gel demonstrates that the front of the peak contains all the indicated subunits.
(D) Structural and topological organization of the 26-subunit kinetochore (rKT26) based on current and previous work (Weir et al., 2016). The drawing is approximately in scale. A crystal structure of the CENP-A nucleosome has been reported previously (Tachiwana et al., 2011). Previously, we determined crystal structures of NDC80CBonsai (an engineered version of the NDC80 complex that retains microtubule-binding and kinetochore localization activities; Ciferri et al., 2008), of the kinetochore-targeting C-terminal domain of KNL1 (KNL1C) (Petrovic et al., 2014), and of the MIS12C (Petrovic et al., 2016), and we used negative-stain EM to obtain a first view in three dimensions of their complex (Petrovic et al., 2014). Shown in orange are experimental molecular models fitted into the EM density. The long axis of the NDC80CBonsai, MIS12C, KNL1 complex is approximately 35 nm, but the length of the actual complex is approximately 90 nm (Huis In 't Veld et al., 2016) due to the extensive coiled-coils of NDC80C that have been trimmed from NDC80CBonsai. This paper adds a view of CCAN to this scheme. CENP-C connects the CENP-A nucleosome, which it binds via a specific binding domain (CENP-BD), to the outer kinetochore, which it binds via a MIS12 binding domain. See also Figure S5.