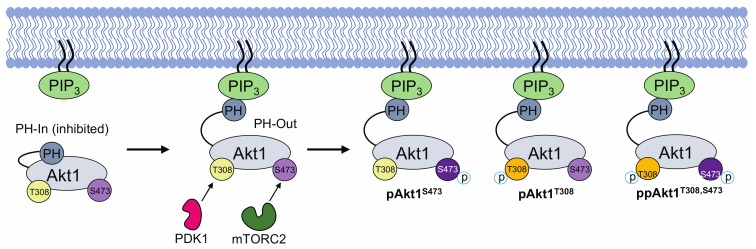
Figure 1.
Simplified schematic of protein kinase B (Akt1) activation via phosphorylation of sites Thr308 and Ser473. The transition from Akt1’s inactive state (PH-in) to its fully active state (ppAkt1T308/S473) requires the release of pleckstrin homology (PH) domain–mediated auto-inhibition. This release occurs when Akt1’s PH domain interacts with PIP3 (PH-out). In the PH-out conformation, Akt1 is more susceptible to phosphorylation at Thr308 and Ser473 by phosphoinositide dependent kinase 1 (PDK1) and mechanistic target of rapamycin complex 2 (mTORC2), respectively. Upon release from PIP3, Akt1 distributes rapidly in the cytosol and translocates to the nucleus to phosphorylate >100 cellular proteins [2,17].