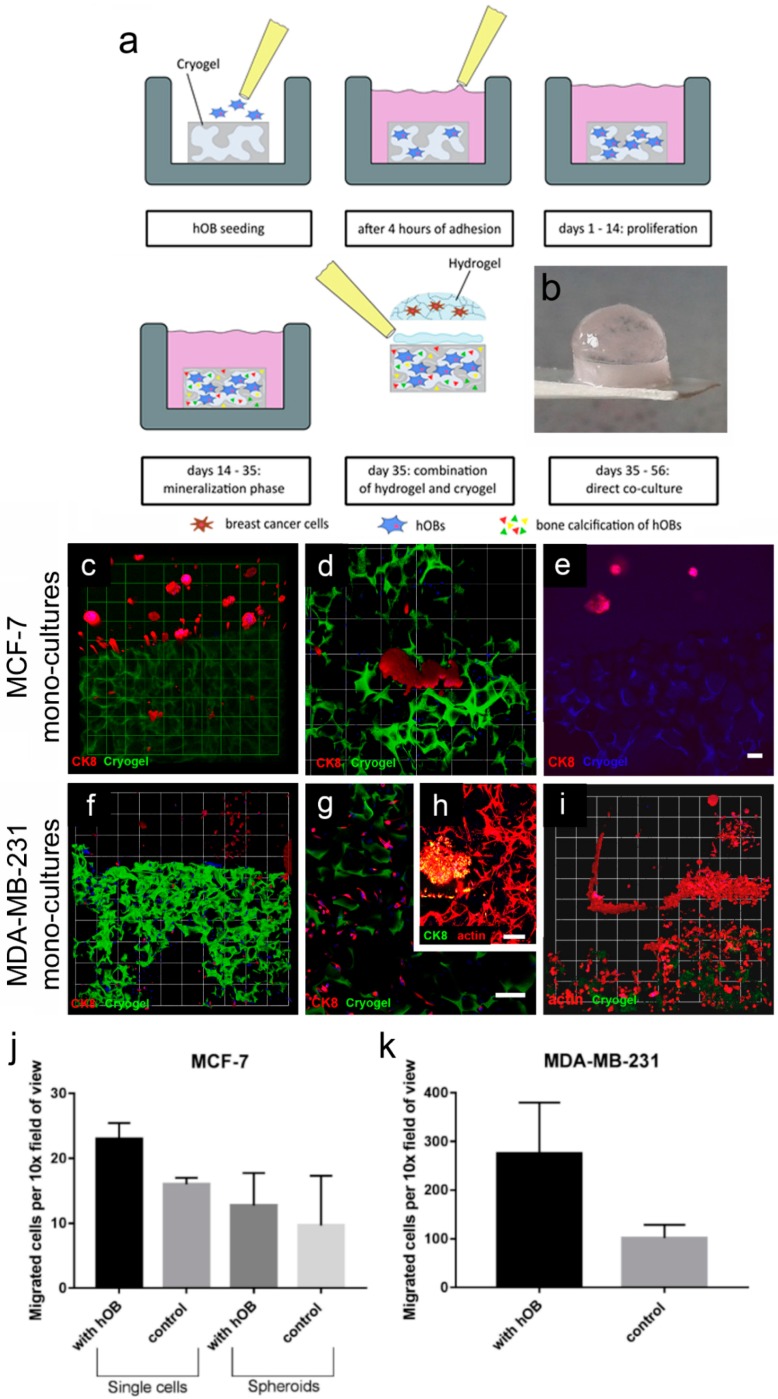
Figure 8.
MCF-7 and MDA-MB-231 monocultures grown in a 3D–3D coculture model. (a) Schematic showing generation of the 3D–3D coculture model: hOBs were seeded onto cylindrical cryogel scaffolds. After 4 h of initial adhesion, 1 mL of hOB growth medium was added to each sample and they were cultured for 14 d. Next, the hOBs were stimulated in the mineralization phase (days 14–35). (b) At 35 d, hydrogels with various cell types were attached on top of the cryogel scaffolds. (c–e) Representative 3D confocal images show MCF-7 monocultures (CK8, red) grown in a hydrogel–cryogel construction and combined with Alexa Fluor 488-labeled cryogels (green) with (c,d) hOB or (e) an empty cryogel control (autofluorescent, blue). (c) A 3D overview of a PEG–MMP hydrogel with tumor spheroids and cryogel with migrated MCF-7 cells (micrometastases) (10×, 1 unit = 155 μm). (d) Micrometastases (1 unit = 77.5 μm). (e) empty cryogel control (blue) without migrated cells. (f) Image shows a 3D overview of a PEG–MMP hydrogel with MDA-MB-231 breast-cancer cells (CK8, red) and cryogel (green) with migrated breast-cancer single cells (10×, 1 unit = 155 µm). (g) Migrated elongated cells within the cryogel (CK8, red; cryogel, green). (h) HOB meshwork and an MDA-MB-231 micrometastasis within the cryogel (actin, red; CK8, green). (i) Migrated MDA-MB-231 cells within the cryogel containing hOBs (CK8, red; cryogel, green). Experiment was performed twice in triplicate (n = 2). Scale bar = 100 µm. (j–k) Migration of MCF-7 and MDA-MB-231 cells to cryogel in a direct coculture model. The numbers of cells are plotted as number of cells/spheroids per 10× field of view. Experiment was performed once in triplicate (n = 1) and data are presented as mean ± SD.