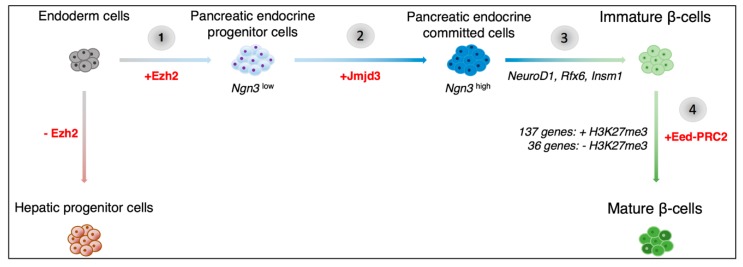
Figure 1.
Schematics of the potential epigenetic mechanisms involved in pancreatic β-cell development. Previous research has shown that the transition from endoderm cells to pancreatic progenitor cells involves the activation of the Enhancer of zeste homolog 2 (Ezh2) histone methyltransferase (1). Subsequently, Neurogenin 3 (Ngn3)low pancreatic endocrine progenitor cells transition to a Ngn3high cell state and are thereby committed to the endocrine cell lineage (2). The histone demethylase, Jumonji domain-containing protein 3 (Jmjd3), was found to be involved in this process. A high level of Ngn3 expression leads to the activation Ngn3 downstream targets, such as Neurogenic differentiation factor 1 (NeuroD1), Regulatory factor X, 6 (Rfx6), and Insulinoma-associated 1 (Insm1) (3). The resulting immature β-cells further develop through the silencing of the immaturity-associated genes (via the addition of H3K27me3 marks) and the de novo activation of maturity-associated genes (with the loss of H3K27me3 marks) (4). The epigenetic changes associated with this stage in development are regulated by the polycomb repressor complex (EeD-PRC2).