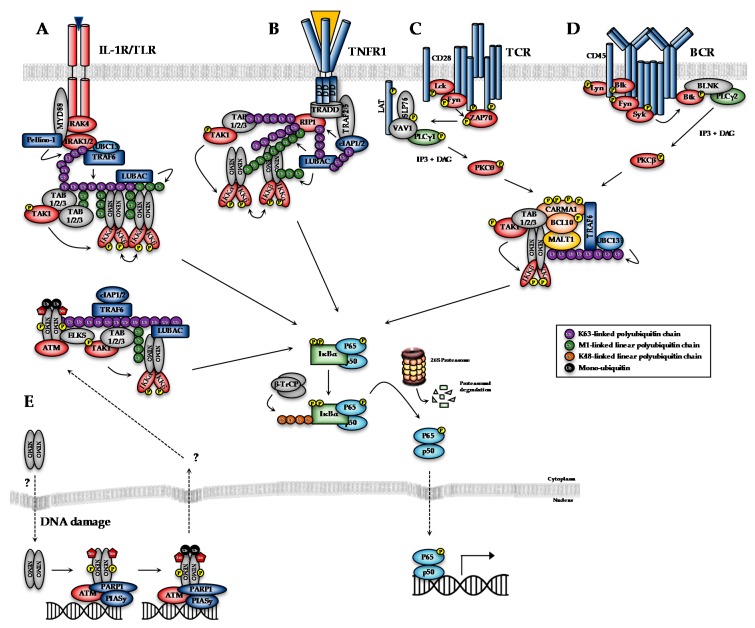
Figure 1.
Overview of canonical and DNA damage-induced NF-κB signalling pathways. (A) Binding of IL-1/Toll-like receptor (TLR) ligands to the interleukin-1 receptor (IL-1R)/TLRs leads to the assembly of the so-called ‘Myddosome’, an oligomeric structure consisting of the adaptor protein MyD88, IL-1 Receptor (IL-1R)-Associated Kinase 4 (IRAK4), IRAK1 and IRAK2. IRAK4 activates IRAK1, allowing IRAK1 to autophosphorylate and subsequently phosphorylate the E3-ligase Pellino-1, which in turn causes K63-polyubiquitylation of IRAK1. This leads to the recruitment and activation of TRAF6, which along with the E2-conjugating complex Ubc13-Uev1a, generates K63-linked polyubiquitin chains that serve to recruit and activate the TAK1 complex or TAB1/2/3-TAK1. K63-linked chains also serve as a substrate for the LUBAC (linear ubiquitin assembly complex) complex, which conjugates M1-linked ubiquitin to these oligomers, to generate M1-K63-linked hybrid ubiquitin chains. The IKK complex is recruited to this complex through interaction of NEMO with M1-linked chains. The co-localisation of TAK1 and IKK to ubiquitin chains leads to activation of the IKK complex, which subsequently phosphorylates IκBα to activate the NF-κB pathway. (B) TNFα binding to the extracellular domain of the receptor leads to the recruitment of TRADD (Tumor necrosis factor receptor type 1-associated DEATH domain) to the cytoplasmic death domains of TNFR1. TRADD, in turn, recruits RIP kinase, and subsequently TRAF2 or TRAF5 adaptor proteins and cIAP1 or cIAP2 to assemble the TNFR1 complex I. cIAP1 and cIAP2 generate K63-linked polubiquitin chains on RIP1 and other components of the complex. This is necessary to recruit LUBAC, which stabilises complex I by catalysing the attachment of linear M1-linked polyubiquitin chains, typically to RIP1. K63-polyubiquitylated RIP1 also recruits the TAK1:TAB complex. LUBAC-mediated M1-linked linear polyubiquitylation of RIP1, meanwhile promotes the recruitment of NEMO, as part of the IKK complex. Membrane proximal recruitment of IKK kinases contributes to IKK activation through proximity to TAK1, which is thought to prime the activation of IKK via phosphorylation of S176/S177 of IKKα/IKKβ, and through oligomerisation of IKK complexes, which is thought to facilitate trans-autophosphorylation of the activation loop, leading to full activation. (C) Engagement of the TCR by a major histocompatibility complex (MHC)-antigen complex leads to recruitment of Src family kinases, including FYN and LCK, which phosphorylate the TCR to promote recruitment of the tyrosine kinase, ZAP-70. ZAP-70 phosphorylates the adapter proteins LAT and SLP-76, which along with VAV1 promote the recruitment and activation of PLCγ1. PLCγ1 generates the second messengers, inositol trisphosphate (IP3) and diacylglycerol (DAG), which, in turn, activate a specific PKC isoform, PKCθ. PKCθ-mediated phosphorylation of CARMA1 triggers a conformational change, enabling CARMA1 to bind to BCL10 and MALT1, to form the CBM complex. BCL10 and MALT1 become polyubiquitinated, possibly through TRAF6 activity, which promotes the recruitment of NEMO, as part of the IKK complex. (D) Antigen binding to BCRs leads to the recruitment and activation of SRC-family kinases, including BLK, LYN, FYN and SYK and adaptors, such as BLNK. This leads to activation of PLCγ2, which catalyses the generation of IP3 and DAG, which ultimately activate a specific PKC isoform, PKCβ. PKCβ phosphorylates CARMA1 to form the CBM complex and ultimately activate the IKK complex. (E) Genotoxic triggers the nuclear accumulation of ‘IKK-free’ NEMO. Within the nucleus NEMO forms a complex with PARP1, PIASy and ATM and undergoes a series of post-translational modification. PIASy promotes the sumoylation of NEMO, which promotes its nuclear localisation. ATM phosphorylates NEMO at Serine 85, which is necessary for the subsequent monoubiquitylation of NEMO. This is thought to trigger the nuclear export of the NEMO-ATM complex, which then, in an ill-defined mechanism, activates TAK1 and the IKK complex. Canonical and DNA damage-induced NF-κB signalling pathways converge at the activation of the IKK complex, which subsequently phosphorylates IκB proteins (at S32 and S36 IκBα). This promotes the recognition of the PEST motif degron within IκBα by β-TrCP, which is part of the E3 ubiquitin ligase SCFβ-TrCP (S phase kinase-associated protein 1 (SKP1)-cullin 1-F-box protein containing β-transducing repeat-containing protein), and its K48-linked ubiquitylation, which targets IκBα for proteasomal degradation. This enables NF-κB complexes (primarily p65-p50 and c-rel/p50 complexes in the case of canonical NF-κB pathways), to accumulate in the nucleus, where they regulate the expression of NF-κB-dependent genes.