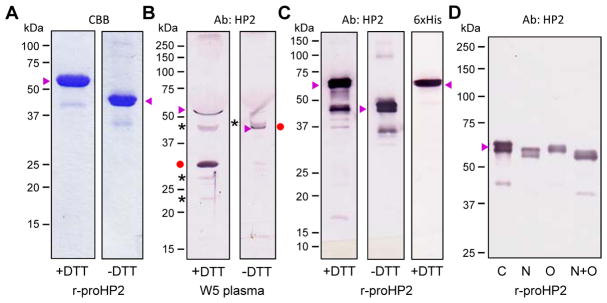
Fig. 3.
Characterization of proHP2 purified from the conditioned medium of Sf9 cells infected by the recombinant baculovirus and mobility comparison with the natural proHP2 and HP2 in the hemolymph. (A) Following cationic exchange and nickel affinity chromatography, 1.0 μg of the purified proHP2 samples were resolved by 10% SDS-PAGE under reducing and non-reducing conditions and stained with Coomassie brilliant blue (CBB). (B) For mobility comparison, 1.0 μl of plasma from day 5, wandering (W5) larvae was treated with 1×SDS sample buffer in the presence or absence of DTT, separated by 10% SDS-PAGE, and detected by 1:1000 diluted HP2 antiserum and enzyme-linked secondary antibody. The proHP2, HP2 catalytic domain, and uncharacterized protein bands are labeled “▶”, “●”, and “*”, respectively. On the right, the HP2 catalytic domain (●) was attached to the light chain and had migrated slower than proHP2 (▶). (C) The gel-separated proHP2 (50 ng, +/− DTT) was transferred onto a nitrocellulose membrane for immunoblot analysis using 1:1000 diluted HP2 (left, middle) or hexahistidine (right) antiserum as the primary antibody. (D) The purified proHP2 was treated with buffer (C for control), PNGase F (N), neuraminidase A and O-glycosidase (O), or all three enzymes (N+O) prior to 7.5% SDS-PAGE and immunoblot analysis. In panels A–D, sizes and positions of the Mr makers are indicated on the left.