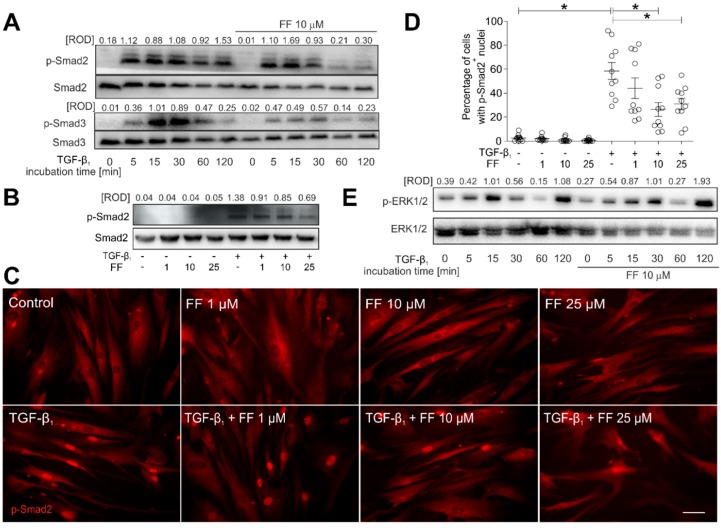
Figure 4.
Fenofibrate affects TGF-β1-dependent Smad signaling pathway activity in HBFs. (A) Cells cultivated in DMEM supplemented with TGF-β1 (5 ng/mL) in the absence or presence of FF (10 μM) for different time points (0–120 min). Then, the cells were lysed, and the total cell lysates were analyzed using immunoblotting. Smad2, Smad3, and their phosphorylated forms were detected using primary antibodies against Smad2, Smad3, phosphorylated (p)-Smad2(Ser465/467) and p-Smad3(Ser423/425), respectively (see Materials and methods; Section 4.7). Representative membranes are shown. Densitometric quantification is presented as values of relative optical densities (ROD) (n = 5) of phospho-Smads in relation to Smads (as control proteins). (B) Immunoblots with relative optical density (ROD) of p-Smad2(Ser467) in relation to Smad2 are presented in HBFs treated by TGF-β1 (5 ng/mL) in the absence or presence of FF (1–25 μM) for 1 h. The results represent mean ± SEM of five independent experiments. (C,D) HBFs (n = 10) cultured in the conditions described in (B) were fixed with 3.7% formaldehyde/PBS, permeabilized, and immunostained for p-Smad2. The fractions of cells with pSmad2+ nuclei were determined using fluorescence microscopy. Representative photos were selected. Scale bar = 25 µm. Data represent the mean ± SEM of ten independent experiments in triplicate. (E) HBFs cultured in the conditions described in (A) were lysed and analyzed by Western blot. Representative immunoblots with relative optical density of p-ERK1/2 in relation to ERK1/2 are presented. Statistical significances were tested using one-way ANOVA with the Bonferroni multiple comparison post hoc Test; * p ≤ 0.05. Note that fenofibrate efficiently attenuates the TGF-β1-induced Smad signaling, but not the ERK-dependent pathway.