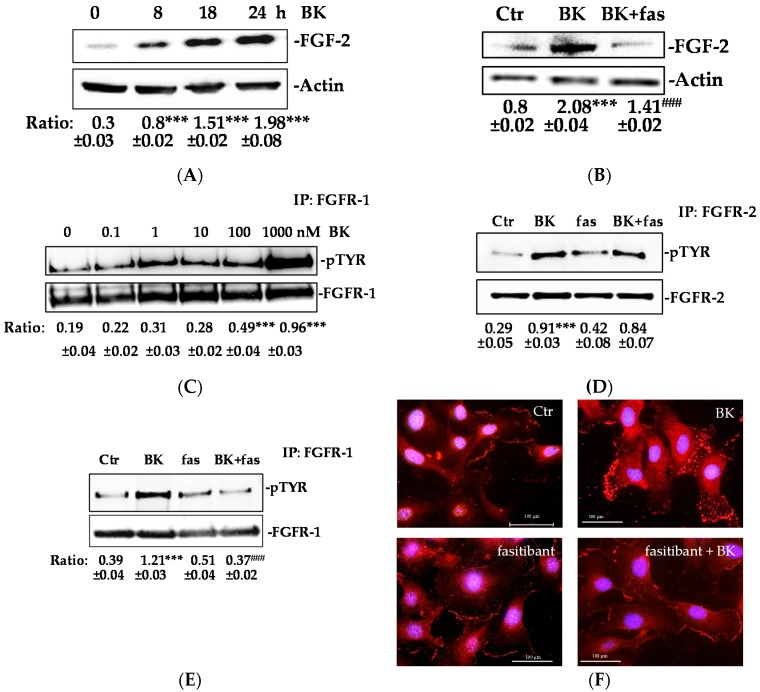
Figure 1.
BK/B2R transactivates FGFR-1 and mediates its internalization. (A) HUVEC were treated with BK (1 μM) for 8, 18, and 24 h, and FGF-2 expression was evaluated using western blot analysis. Results were normalized to actin. Quantification was expressed as an arbitrary density unit (ADU). The results presented are representative of three independent experiments (n = 3) with similar results. (B) HUVEC were treated with fasitibant (fas, 1 µM, 30 min), then stimulated with BK (1 μM) for 24 h, and FGF-2 expression was evaluated using western blot analysis. Results were normalized to actin. (C) HUVEC were treated with BK (0.1–1000 nM, 10 min), FGFR-1 was immunoprecipitated (IP), and its activation was investigated by anti-pTYR antibody. Results were normalized to FGFR-1. (D,E) HUVEC were treated with fasitibant (fas, 1 µM, 30 min), then stimulated with 1 μM BK (10 min), FGFR-2 and FGFR-1 were immunoprecipitated (IP), and its activation was investigated by anti-pTYR antibody. Results were normalized to FGFR-2 and FGFR-1, respectively. *** p < 0.001 vs. Ctr; ### p < 0.001 vs. BK treated cells. Ctr (control, 0.1% FBS). (F) Immunofluorescence analysis of FGFR-1 localization in endothelial cells in the control condition (Ctr, 0.1% FBS) and in the presence of BK (1 μM, 10 min) alone or in combination with fasitibant (1 μM). Magnification, 100×, scale bar = 100 μm.