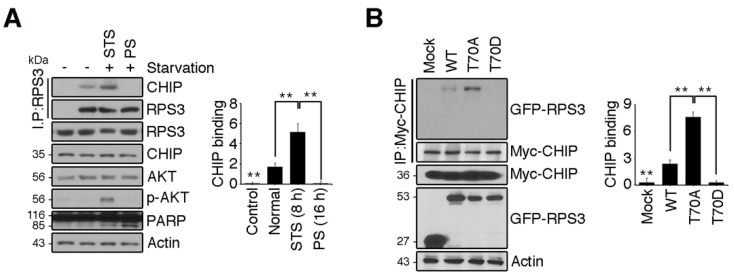
Figure 5.
RPS3 degradation is regulated by HSP70/CHIP under starvation conditions. (A) HEK 293T cells were incubated with or without FBS. Endogenous RPS3 and CHIP binding affinities were determined by means of anti-RPS3 and anti-CHIP antibodies after immunoprecipitation with the anti-RPS3 antibody. (B) GFP-RPS3 WT, T70A, T70D, or control vector were transfected into HEK 293T cells with Myc-CHIP. Protein lysates were subjected to immunoprecipitation with the anti-Myc antibody, and binding affinities with RPS3 mutant forms were determined by immunoblotting with an anti-GFP antibody. Western blot intensities were measured by Image J and their values were normalized with normal cell lysate (A) or mock transfected cell (B). Values in this figure represent the mean ± SEM from three independent experiments, and the images shown are representative of at least three independent experiments. Statistical significance was determined using a one-way ANOVA test followed by Turkey’s post-test (* p < 0.01; ** p < 0.001).